Nanocellulose is the most abundant material extracted from plants, animals, and bacteria. Nanocellulose is a cellulosic material with nano-scale dimensions and exists in the form of cellulose nanocrystals (CNC), bacterial nanocellulose (BNC), and nano-fibrillated cellulose (NFC). Owing to its high surface area, non-toxic nature, good mechanical properties, low thermal expansion, and high biodegradability, it is obtaining high attraction in the fields of electronics, paper making, packaging, and filtration, as well as the biomedical industry. To obtain the full potential of nanocellulose, it is chemically modified to alter the surface, resulting in improved properties.
- nanocellulose
- biodegradable
- nanocomposites
- chemical functionalization
- extraction
- applications
1. Introduction
2. Nanocellulose and Its Various Sources
| Nanocellulose Types | Sources | Extraction Method and Size |
|---|---|---|
| CNC | Cotton, tunicin, mulberry bark, hemp, wood, wheat straw. | Acid hydrolysis 5–70 nm in diameter 100–250 nm in length |
| BNC | Sugars and alcohols. | Extracted from bacterial synthesis 20–100 nm in diameter |
| NFC | Wood, hemp, flax, potato tuber, sugar beet. | A mechanical method of breaking the cellulose 5–60 nm in diameter |
3. Nanocellulose Extraction Processes
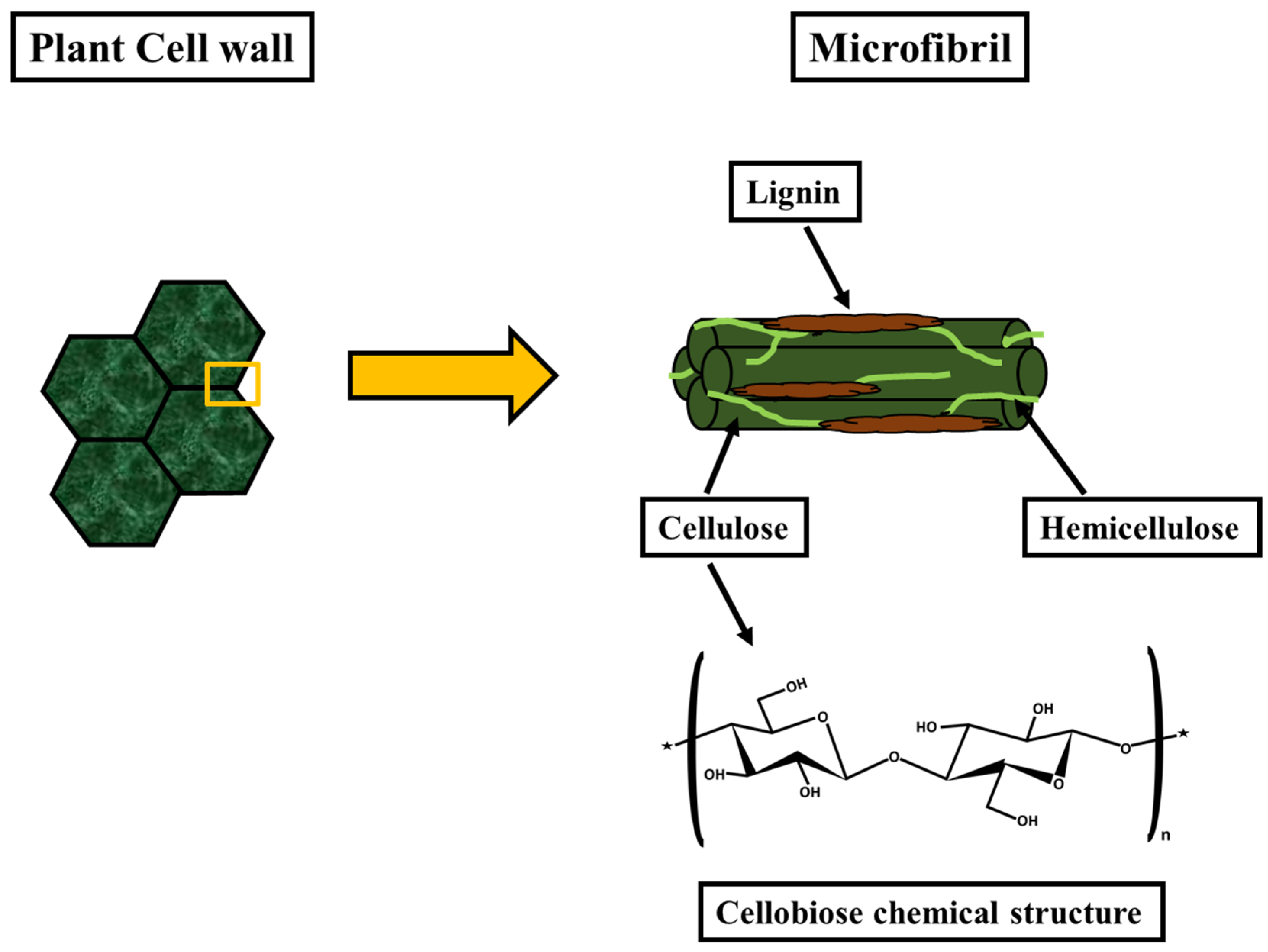
3.1. Biomass Treatment for Nanocellulose Extraction
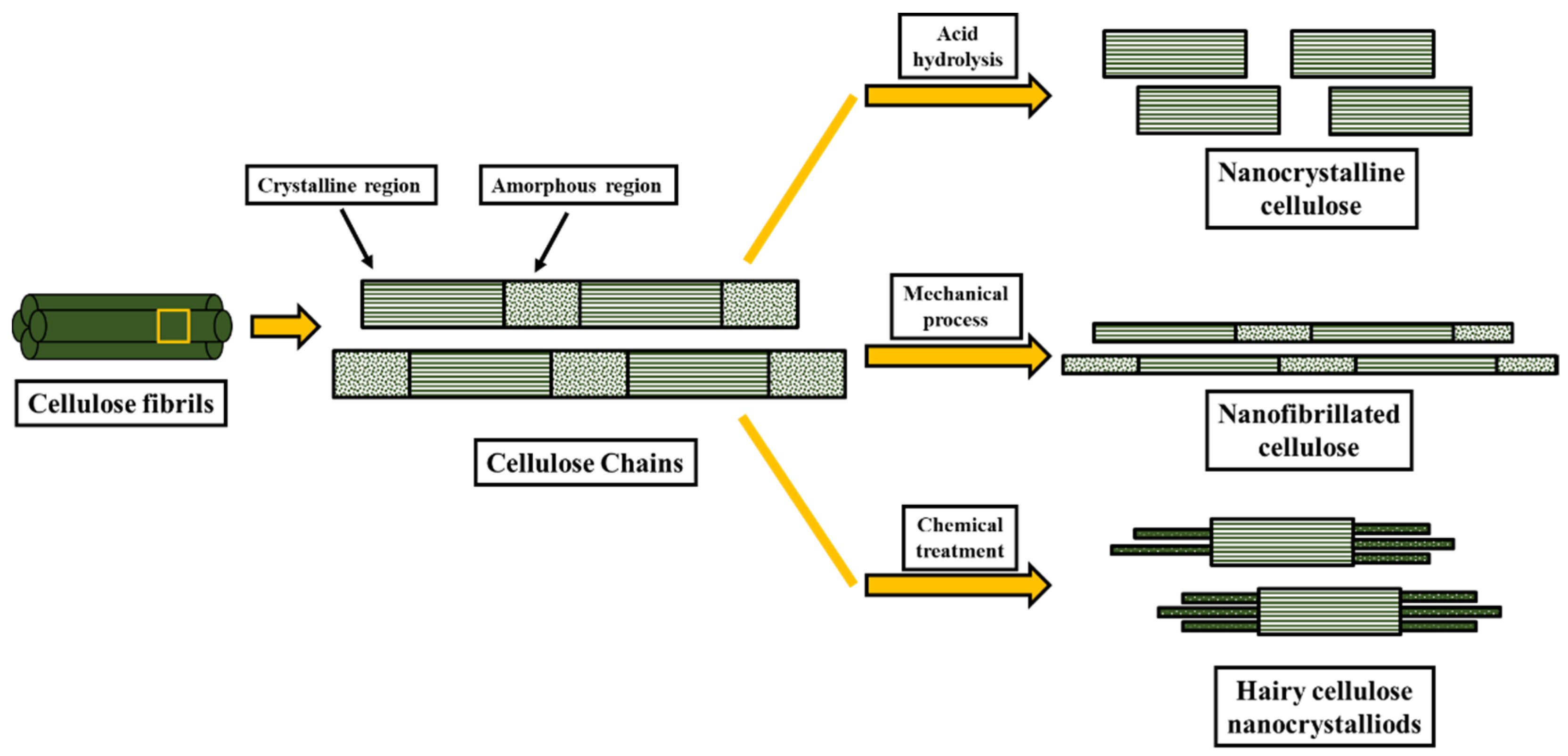
3.2. Nanocellulose Isolation
| Biological Methods | Mechanical Methods | Chemical Methods |
|---|---|---|
| Fungi treatment | Steam explosion | Ionic treatment |
| Bacteria treatment | Ball milling | Alkaline treatment |
| Enzymatic hydrolysis | Disintegration | Acid hydrolysis |
| Grinding | Oxidation | |
| Electrospinning | Solvent extraction | |
| Ultrasonication | ||
| Homogenization |
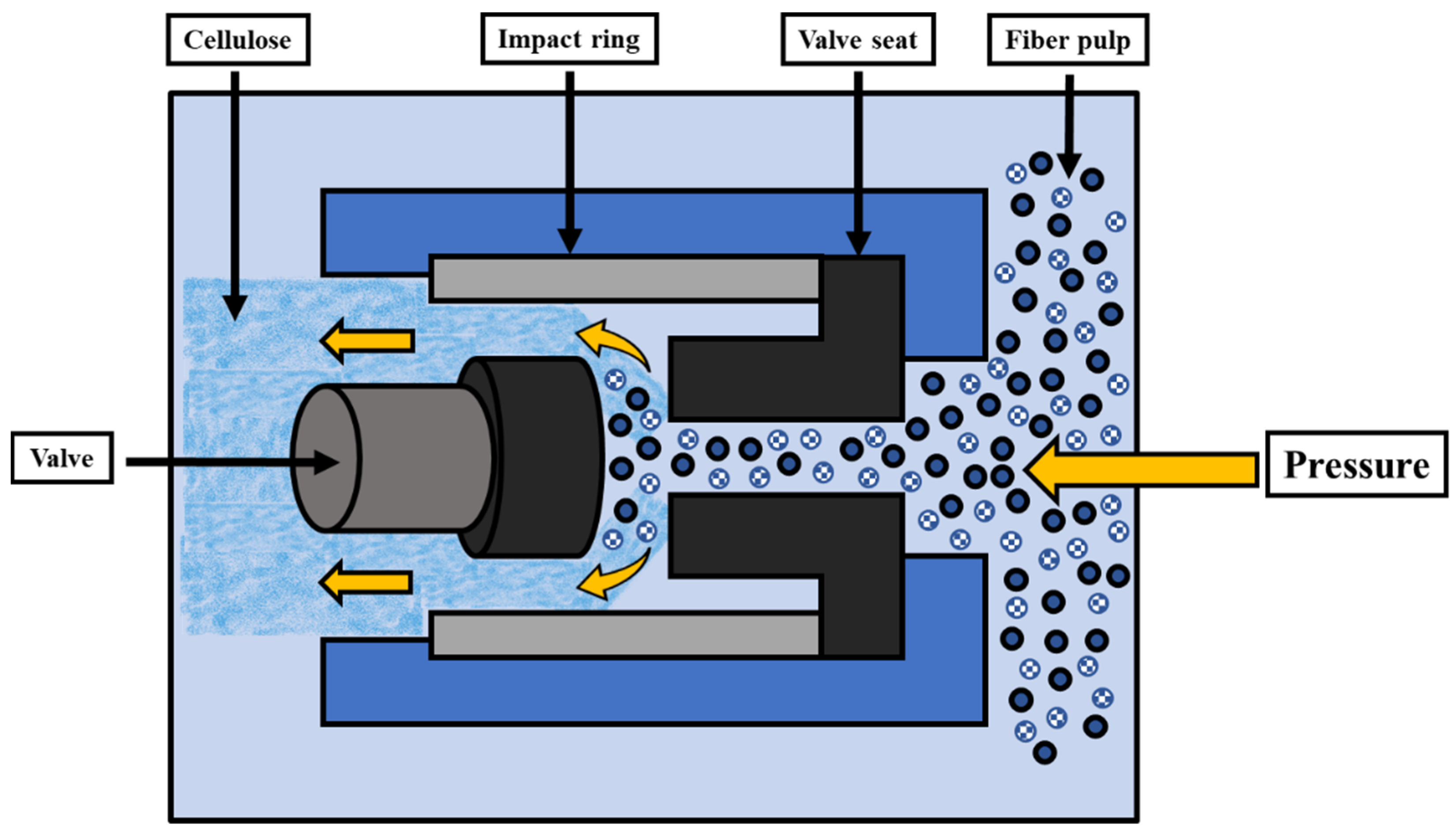
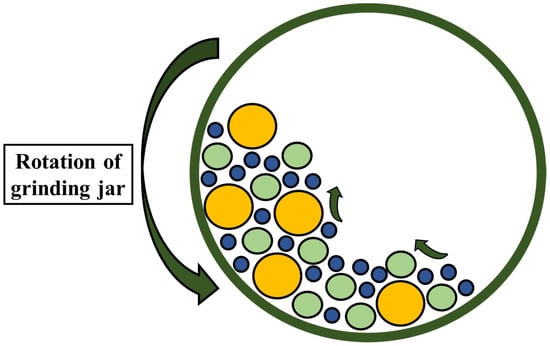
3.3. BNC Extraction
This entry is adapted from the peer-reviewed paper 10.3390/polym14214468
References
- Karim, M.R.A.; Tahir, D.; Haq, E.U.; Hussain, A.; Malik, M.S. Natural fibres as promising environmental-friendly reinforcements for polymer composites. Polym. Polym. Compos. 2020, 29, 277–300.
- Tan, K.W.; Heo, S.K.; Foo, M.L.; Chew, I.M.L.; Yoo, C.K. An insight into nanocellulose as soft condensed matter: Challenge and future prospective toward environmental sustainability. Sci. Total Environ. 2019, 650, 1309–1326.
- Dufresne, A. Nanocellulose: A new ageless bionanomaterial. Mater. Today 2013, 16, 220–227.
- Lee, K.-Y.; Aitomäki, Y.; Berglund, L.A.; Oksman, K.; Bismarck, A. On the use of nanocellulose as reinforcement in polymer matrix composites. Compos. Sci. Technol. 2014, 105, 15–27.
- Gabriel, T.; Belete, A.; Syrowatka, F.; Neubert, R.H.H.; Gebre-Mariam, T. Extraction and characterization of celluloses from various plant byproducts. Int. J. Biol. Macromol. 2020, 158, 1248–1258.
- Fatima, A.; Yasir, S.; Ul-Islam, M.; Kamal, T.; Ahmad, M.W.; Abbas, Y.; Manan, S.; Ullah, M.W.; Yang, G. Ex situ development and characterization of green antibacterial bacterial cellulose-based composites for potential biomedical applications. Adv. Compos. Hybrid Mater. 2022, 5, 307–321.
- Abdul Karim, M.R.; Tahir, D.; Hussain, A.; Ul Haq, E.; Khan, K.I. Sodium carbonate treatment of fibres to improve mechanical and water absorption characteristics of short bamboo natural fibres reinforced polyester composite. Plast. Rubber Compos. 2020, 49, 425–433.
- Abdul Karim, M.R.; Tahir, D.; Khan, K.I.; Hussain, A.; Haq, E.U.; Malik, M.S. Improved mechanical and water absorption properties of epoxy-bamboo long natural fibres composites by eco-friendly Na2CO3 treatment. Plast. Rubber Compos. 2022, 22, 1–14.
- Dufresne, A. Potential of nanocellulose as a reinforcing phase for polymers. J. Sci. Technol. For. Prod. Process. 2012, 2, 6–16.
- Moon, R.J.; Martini, A.; Nairn, J.; Simonsen, J.; Youngblood, J. Cellulose nanomaterials review: Structure, properties and nanocomposites. Chem. Soc. Rev. 2011, 40, 3941–3994.
- Lavoine, N.; Desloges, I.; Dufresne, A.; Bras, J. Microfibrillated cellulose—Its barrier properties and applications in cellulosic materials: A review. Carbohydr. Polym. 2012, 90, 735–764.
- Abdul Khalil, H.P.S.; Bhat, A.H.; Ireana Yusra, A.F. Green composites from sustainable cellulose nanofibrils: A review. Carbohydr. Polym. 2012, 87, 963–979.
- Ferrer, A.; Pal, L.; Hubbe, M. Nanocellulose in packaging: Advances in barrier layer technologies. Ind. Crops Prod. 2017, 95, 574–582.
- Klemm, D.; Kramer, F.; Moritz, S.; Lindström, T.; Ankerfors, M.; Gray, D.; Dorris, A. Nanocelluloses: A New Family of Nature-Based Materials. Angew. Chem. Int. Ed. 2011, 50, 5438–5466.
- Dufresne, A. Chapter 1. Nanocellulose: Potential Reinforcement in Composites. In Natural Polymers: Volume 2: Nanocomposites; Maya, J., John, S.T., Eds.; RSC Publishing: Cambridge, UK, 2012; pp. 1–32.
- Abitbol, T.; Rivkin, A.; Cao, Y.; Nevo, Y.; Abraham, E.; Ben-Shalom, T.; Lapidot, S.; Shoseyov, O. Nanocellulose, a tiny fiber with huge applications. Curr. Opin. Biotechnol. 2016, 39, 76–88.
- Nechyporchuk, O.; Belgacem, M.N.; Bras, J. Production of cellulose nanofibrils: A review of recent advances. Ind. Crops Prod. 2016, 93, 2–25.
- Jozala, A.F.; de Lencastre-Novaes, L.C.; Lopes, A.M.; de Carvalho Santos-Ebinuma, V.; Mazzola, P.G.; Pessoa, A., Jr.; Grotto, D.; Gerenutti, M.; Chaud, M.V. Bacterial nanocellulose production and application: A 10-year overview. Appl. Microbiol. Biotechnol. 2016, 100, 2063–2072.
- Manan, S.; Ullah, M.W.; Ul-Islam, M.; Shi, Z.; Gauthier, M.; Yang, G. Bacterial cellulose: Molecular regulation of biosynthesis, supramolecular assembly, and tailored structural and functional properties. Prog. Mater. Sci. 2022, 129, 100972.
- Haafiz, M.K.M.; Hassan, A.; Zakaria, Z.; Inuwa, I.M. Isolation and characterization of cellulose nanowhiskers from oil palm biomass microcrystalline cellulose. Carbohydr. Polym. 2014, 103, 119–125.
- Sharma, A.; Thakur, M.; Bhattacharya, M.; Mandal, T.; Goswami, S. Commercial application of cellulose nano-composites—A review. Biotechnol. Rep. 2019, 21, e00316.
- Sharma, A.; Mandal, T.; Goswami, S. Cellulose nanofibers from rice straw: Process development for improved delignification and better crystallinity index. Trends Carbohydr. Res. 2017, 9, 16–27.
- Dos Santos, R.M.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Dantas, N.O.; Pasquini, D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind. Crops Prod. 2013, 50, 707–714.
- Phanthong, P.; Ma, Y.; Guan, G.; Abudula, A. Extraction of Nanocellulose from Raw Apple Stem. J. Jpn. Inst. Energy. 2015, 94, 787–793.
- Kargarzadeh, H.; Ioelovich, M.; Ahmad, I.; Thomas, S.; Dufresne, A. Methods for Extraction of Nanocellulose from Various Sources. In Handbook of Nanocellulose and Cellulose Nanocomposites; Kargarzadeh, H., Ahmad, I., Thomas, A.D.S., Eds.; John Wiley & Sons, Ltd.: Weinheim, Germany, 2017; pp. 1–49.
- Bondeson, D.; Mathew, A.; Oksman, K. Optimization of the isolation of nanocrystals from microcrystalline cellulose by acid hydrolysis. Cellulose 2006, 13, 171–180.
- Dong, X.M.; Revol, J.F.; Gray, D.G. Effect of microcrystallite preparation conditions on the formation of colloid crystals of cellulose. Cellulose 1998, 5, 19–32.
- Maiti, S.; Jayaramudu, J.; Das, K.; Reddy, S.M.; Sadiku, R.; Ray, S.S.; Liu, D. Preparation and characterization of nano-cellulose with new shape from different precursor. Carbohydr. Polym. 2013, 98, 562–567.
- Das, K.; Ray, D.; Bandyopadhyay, N.R.; Ghosh, T.; Mohanty, A.K.; Misra, M. A study of the mechanical, thermal and morphological properties of microcrystalline cellulose particles prepared from cotton slivers using different acid concentrations. Cellulose 2009, 16, 783–793.
- Iranmahboob, J.; Nadim, F.; Monemi, S. Optimizing acid-hydrolysis: A critical step for production of ethanol from mixed wood chips. Biomass Bioenergy 2002, 22, 401–404.
- Hamelinck, C.N.; Van Hooijdonk, G.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410.
- Wang, N.; Ding, E.; Cheng, R. Thermal degradation behaviors of spherical cellulose nanocrystals with sulfate groups. Polymer 2007, 48, 3486–3493.
- Abdul Khalil, H.P.S.; Davoudpour, Y.; Islam, M.N.; Mustapha, A.; Sudesh, K.; Dungani, R.; Jawaid, M. Production and modification of nanofibrillated cellulose using various mechanical processes: A review. Carbohydr. Polym. 2014, 99, 649–665.
- Phanthong, P.; Reubroycharoen, P.; Hao, X.; Xu, G.; Abudula, A.; Guan, G. Nanocellulose: Extraction and application. Carbon Resour. Convers. 2018, 1, 32–43.
- Wahlström, R.M.; Suurnäkki, A. Enzymatic hydrolysis of lignocellulosic polysaccharides in the presence of ionic liquids. Green Chem. 2015, 17, 694–714.
- Moniruzzaman, M.; Ono, T. Separation and characterization of cellulose fibers from cypress wood treated with ionic liquid prior to laccase treatment. Bioresour. Technol. 2013, 127, 132–137.
- Li, J.; Wei, X.; Wang, Q.; Chen, J.; Chang, G.; Kong, L.; Su, J.; Liu, Y. Homogeneous isolation of nanocellulose from sugarcane bagasse by high pressure homogenization. Carbohydr. Polym. 2012, 90, 1609–1613.
- Wang, Y.; Bian, K.; Hu, C.; Zhang, Z.; Chen, N.; Zhang, H.; Qu, L. Flexible and wearable graphene/polypyrrole fibers towards multifunctional actuator applications. Electrochem. Commun. 2013, 35, 49–52.
- Väntsi, O.; Kärki, T. Utilization of recycled mineral wool as filler in wood-polypropylene composites. Constr. Build. Mater. 2014, 55, 220–226.
- Zhou, Y.M.; Fu, S.Y.; Zheng, L.M.; Zhan, H.Y. Effect of nanocellulose isolation techniques on the formation of reinforced poly(vinyl alcohol) nanocomposite films. Express Polym. Lett. 2012, 6, 794–804.
- Filson, P.B.; Dawson-Andoh, B.E. Sono-chemical preparation of cellulose nanocrystals from lignocellulose derived materials. Bioresour. Technol. 2009, 100, 2259–2264.
- Tang, L.; Huang, B.; Lu, Q.; Wang, S.; Ou, W.; Lin, W.; Chen, X. Ultrasonication-assisted manufacture of cellulose nanocrystals esterified with acetic acid. Bioresour. Technol. 2013, 127, 100–105.
- Ago, M.; Endo, T.; Hirotsu, T. Crystalline transformation of native cellulose from cellulose I to cellulose II polymorph by a ball-milling method with a specific amount of water. Cellulose 2004, 11, 163–167.
- Barakat, A.; Mayer-Laigle, C.; Solhy, A.; Arancon, R.A.D.; De Vries, H.; Luque, R. Mechanical pretreatments of lignocellulosic biomass: Towards facile and environmentally sound technologies for biofuels production. RSC Adv. 2014, 4, 48109–48127.
- Kim, H.J.; Lee, S.; Kim, J.; Mitchell, R.J.; Lee, J.H. Environmentally friendly pretreatment of plant biomass by planetary and attrition milling. Bioresour. Technol. 2013, 144, 50–56.
- Baheti, V.; Abbasi, R.; Militky, J. Ball milling of jute fibre wastes to prepare nanocellulose. World J. Eng. 2012, 9, 45–50.
- Feng, Y.T.; Han, K.; Owen, D.R.J. Discrete element simulation of the dynamics of high energy planetary ball milling processes. Mater. Sci. Eng. A 2004, 375, 815–819.
- Avolio, R.; Bonadies, I.; Capitani, D.; Errico, M.E.; Gentile, G.; Avella, M. A multitechnique approach to assess the effect of ball milling on cellulose. Carbohydr. Polym. 2012, 87, 265–273.
- Ago, M.; Endo, T.; Okajima, K. Effect of solvent on morphological and structural change of cellulose under ball-milling. Polym. J. 2007, 39, 435–441.
- Brown, E.E.; Laborie, M.P.G. Bioengineering bacterial cellulose/poly(ethylene oxide) nanocomposites. Biomacromolecules 2007, 8, 3074–3081.
- Choi, S.M.; Shin, E.J. The nanofication and functionalization of bacterial cellulose and its applications. Nanomaterials 2020, 10, 406.
- Ullah, M.W.; Ul-Islam, M.; Khan, S.; Kim, Y.; Park, J.K. Innovative production of bio-cellulose using a cell-free system derived from a single cell line. Carbohydr. Polym. 2015, 132, 286–294.
