Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The use of Xenopus oocytes in electrophysiological and biophysical research constitutes a long and successful story, providing major advances to the knowledge of the function and modulation of membrane proteins, mostly receptors, ion channels, and transporters. These cells are capable of correctly expressing heterologous proteins after injecting the corresponding mRNA or cDNA. The Xenopus oocyte has become an outstanding host–cell model to carry out detailed studies on the function of fully-processed foreign membrane proteins after their microtransplantation to the oocyte.
- Xenopus oocytes
- membrane protein biophysics
- neuroreceptor microtransplantation
- proteoliposomes
- membrane protein-lipid interactions
1. Background
Xenopus laevis, the South African clawed frog, is a laboratory animal that is especially suitable for diverse biological studies since, unlike other anurans, their ovaries always contain oocytes in all stages of development (Figure 1) and new cycles of oogenesis can be initiated during any season by hormonal treatment. In the last five decades, the use of Xenopus oocytes has grown exponentially and has become a chief tool in many laboratories around the world in studies dealing with molecular, cellular, and developmental biology [1][2][3][4][5]. In fact, since the last decade of the 20th century, the Xenopus oocyte constitutes one of the most widely used cell model in molecular biology [3][4]. Furthermore, Xenopus oocytes are particularly useful cells to carry out electrophysiological recordings to study both native ion channels and receptors [6][7] (reviewed in [2][8][9][10]) and foreign proteins transplanted to this convenient host cell after the injection of either the corresponding mRNA or lipid vesicles containing the purified protein (reviewed in [2][9][11][12][13]). Xenopus oocytes have also become the reference model for biophysical studies dealing with structure to function relationships, addressed to unravel the functional consequences of specific mutations of membrane receptors, channels, and transporters (reviewed by [13][14]). Moreover, transplanting foreign, fully-processed membrane proteins to Xenopus oocytes is a methodology more recently used to look deeper into the role played by ion channels and neuroreceptors in the pathogenesis of different diseases as well as for drug screening using electrophysiological recordings and/or fluorescence assays [13][15][16]. Altogether, this has led to the Xenopus oocyte being regarded as a “living test tube” [17].
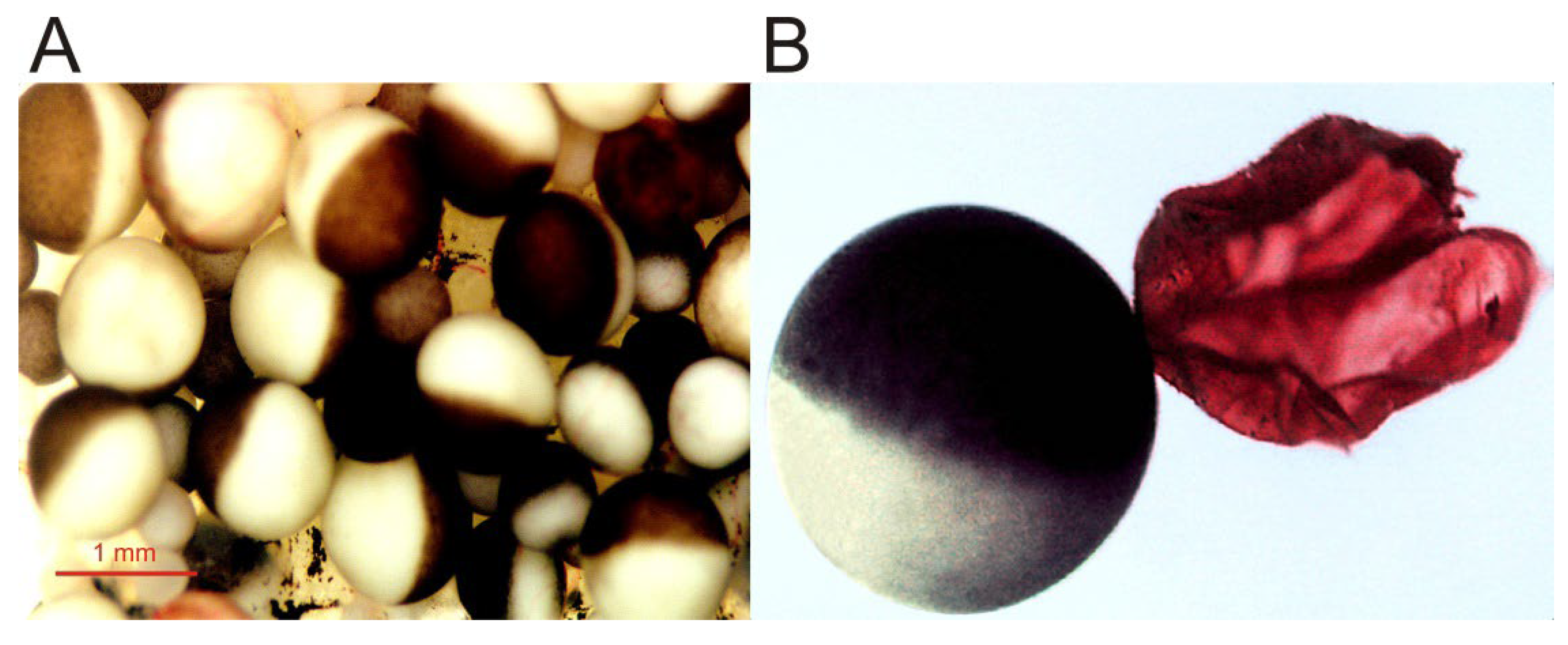
Figure 1. Micrographs of Xenopus oocytes. (A) Image of an opened and extended fragment of the Xenopus ovary lobule, showing oocytes at different stages of development. (B) Full-grown, immature oocyte, and the plasma membrane (together with its vitelline envelope) from another oocyte, manually isolated and stained with ink for better observation.
2. Advantages and Drawbacks of Using Xenopus Oocytes to the Study of Membrane Proteins
As stated by August Krogh [18], an outstanding physiologist awarded the Nobel Prize, for every biological problem there is an organism (model) on which it can be most conveniently studied. In this sense, the Xenopus oocyte model, introduced by Ricardo Miledi, constitutes an excellent paradigm of the Krogh principle to carry out detailed studies on the function of foreign membrane proteins, as was the squid giant axon to understand the ionic basis of the action potential, the frog end-plate junction and the squid stellate ganglion to unveil the basis of synaptic transmission or the Aplysia ganglia, which allowed for the molecular mechanisms of learning and memory to be dealt with. Some of the advantages that the oocyte fulfills to study foreign proteins are:
- (a)
-
Xenopus oocytes have a continuous and asynchronous development, allowing a year-round use of these cells [1].
- (b)
-
Large size, up to 1.3 mm, and roughly spherical shape [1], which facilitates electrophysiological recordings, even allowing the introduction of additional micropipettes for microinjecting compounds [2]. Furthermore, its size enables simultaneous biochemical and optical techniques with quite high spatio-temporal discrimination [19].
- (c)
- (d)
-
These cells are easy to manage and cheap to maintain. Moreover, these cells can be kept alive in an inorganic buffer for long periods (up to several weeks) after their isolation from the ovary, without the need of a specific sterile serum or medium [2][14][16]. Nevertheless, oocytes progressively uncouple from the surrounding follicular cells after their separation from the ovary, losing responsiveness to certain hormones and neurotransmitters.
- (e)
- (f)
-
The oocyte plasma membrane can be quite easily isolated either manually ([30][31]; see Figure 1) or by simple biochemical procedures [32]. The isolation of plasma membranes enables one to determine the presence of the microtransplanted protein at the oocyte plasma membrane [31][33] and allows studies concerning both the targeting of membrane proteins and the quantitation of the ratios between functional and the total number of ion channels/receptors incorporated into the cell membrane [12][33][34].
- (g)
-
Although the membrane of Xenopus oocytes might present certain ion channels and receptors, the expression of endogenous proteins from their own mRNA is low (about 5% of their stored mRNA, [13]). Thus the oocyte plasma membrane usually lacks significant levels of neuronal voltage-dependent Na+ and K+ channels and many neuroreceptors and transporters [10].
- (h)
-
The oocyte has powerful intracellular signaling cascades, mostly involving InsP3 synthesis and Ca2+ release from intracellular stores after phospholipase-C activation. Thus, heterologous expression of certain metabotropic receptors can be simply monitored by recording Ca2+-dependent Cl− currents, since the Ca2+-dependent Cl− channel, anoctamin 1 (TMEM16A), is highly expressed in the oocyte membrane [2][27][35][36].
- (i)
Nevertheless, Xenopus oocytes also present some disadvantages when studying membrane proteins including:
- (a)
-
In the ovary, oocytes are found as follicles, constituted by several cellular and acellular layers surrounding the oocyte, from inner to outer: (i) the oocyte; (ii) a fibrous vitelline membrane; (iii) a monolayer of follicular cells, which is coupled to microvilli of the oocyte membrane by gap junctions [37]; (iv) a theca, containing mainly collagen, fibrocytes and small blood vessels; and (v) a layer of epithelial cells. The layers surrounding the oocyte might constitute a handicap to study certain transplanted proteins since follicular cells express ion channels and receptors of their own and are electrically coupled to the oocyte. However, the layers surrounding the oocyte can be removed either manually or by enzymatic means.
- (b)
-
There is a certain variability in the expression efficiency of oocytes, which has been related to seasonal differences [27]. Actually, some laboratories stop working with oocytes during summer because of their low quality. Furthermore, noise and other vibrations should be restricted around aquarium facilities since Xenopus are quite sensitive to them.
- (c)
-
As previously mentioned, oocytes randomly express certain ion channels and receptors, which might be confused with those heterologously expressed [13]. Thus, it becomes necessary to keep in mind the channels and receptors that can be endogenously expressed by oocytes (excellent reviews regarding this issue are provided in [2][8][9][10][24][25]), though some of them can be pharmacologically blocked. Nevertheless, other cell models commonly used for the heterologous expression of ion channels and transporters such as human embryonic kidney cells (HEK) also have endogenous ion channels [38].
- (d)
-
The large size of the oocyte together with the presence of several surrounding layers (cellular and acellular) constitute a limitation to obtaining fast ligand-applications, particularly when using large molecules [39], and consequently, to record fast-kinetics currents (it hinders the resolution of ligand-elicited currents at resolutions below hundred milliseconds; [40]). Moreover, when follicular cells remain attached to the oocyte, certain measured pharmacological values (i.e., half maximal effective concentration, EC50, or half maximal inhibitory concentration, IC50) might be inaccurate [39]. In addition, several factors such as the oocyte large size, the presence of numerous microvilli at its plasma membrane, and its electrical coupling to follicular cells contribute to eliciting extremely large capacitive artifacts, which prevent resolving fast voltage-dependent currents below a few milliseconds [14].
3. Transplant of Fully Processed Membrane Proteins to the Xenopus Oocyte
As previously indicated, Xenopus oocytes have allowed for the biophysical characterization of many ion channels, neurotransmitter receptors, and transporters thanks to their ease of use, amenability for electrophysiological recordings and their capability to efficiently and faithfully translate most heterologous mRNAs (reviewed in [2][24][25]). It is known that Xenopus oocytes are able to make many post-translational modifications of the proteins coded by the exogenous mRNA (as glycosylation, phosphorylation, acetylation, or folding), and to correctly assemble oligomeric receptor/channel complexes. However, occasionally, they do not mimic the post-translational modifications carried out by the cells that used to express them natively. Most likely, these differences account for the failed or altered function of some foreign proteins expressed in oocytes and, perhaps, also for the variability observed between oocytes [27]. For instance, Torpedo nicotinic acetylcholine (ACh) receptors (nAChRs) expressed in oocytes after injecting the corresponding mRNAs have an altered pattern of glycosylation [41], and neuronal sympathetic nAChRs (α3β4 and α4β4 subunits) do not exhibit certain properties of the native receptors, putatively because oocytes fail to correctly assemble their different subunits or because of the post-translational channel modifications [42]. Similarly, native brain and heterologously expressed rat α4β2 nAChRs showed significant pharmacological differences [43]. In this regard, it should be considered that certain membrane proteins including ion channels are modulated by their interaction with accessory subunits, which might be lacking in the host cell membrane and are not incorporated when mRNA is used to express specific foreign proteins in the oocyte [13][27][43][44]. Additionally, specific lipid requirements of certain membrane proteins might constitute a severe handicap to the faithful functional expression of some heterologous proteins [45]. Moreover, it has been shown that different mutations in the M4 (the outermost lipid-facing α-helix of the transmembrane domain) of α4β2 nAChRs reduced or abolished the function of these receptors when expressed in the HEK cells, but not when expressed in Xenopus oocytes [46]. The differences in the functional activity of these mutant nAChRs between both cell types seem to be due to the different lipid composition of their respective plasma membranes [46].
The above-mentioned handicaps were overcome when Marsal et al. [47] found that foreign membranes carrying neurotransmitter receptors and ion channels could be incorporated into the Xenopus oocyte membrane, which allowed for the microtransplantation of fully processed proteins retaining their natural environment. Since then, several groups have followed this approach of the intracellular injection of plasma membranes to study foreign membrane proteins in Xenopus oocytes (reviewed in [12]). Thus, many neuroreceptors, transporters, and ion channels have been transplanted from their original cells to the oocyte membrane including: (i) nAChRs from Torpedo electroplaques [33][47], muscle fibers [48][49], brain neurons [50], and from cell lines overexpressing heterologous neuronal nAChRs [26]; (ii) gamma-aminobutyric acid receptor type A (GABAAR) from the brain synaptosomal membranes [15][26][51][52][53][54][55][56][57][58][59][60][61]; (iii) glutamate (AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid/Kainate/NMDA, N-methyl-D-aspartate) receptors [26][52][62][63][64]; (iv) voltage-activated Na+ and Ca2+ channels from the human brain [34][65]; (v) Cl− channels from Torpedo electroplax [33][47] and from human syncytiotrophoblast microvillous membranes [66]; and (vi) membrane transporters such as P-glycoprotein [31] and the Cl− transporters, KCC1 (K+–Cl− cotransporter type 1) and NKCC2 (Na+–K+–Cl− cotransporter type 2; [55]).
The intracellular injection of plasma membranes to insert foreign proteins into the oocyte membrane was later extended by microtransplanting functional proteins after their purification and reconstitution in lipid vesicles of defined composition [33][67][68][69]. The main steps followed to microtransplant purified and reconstituted nAChRs from Torpedo electroplaques to the Xenopus oocyte membrane are shown in Figure 2.
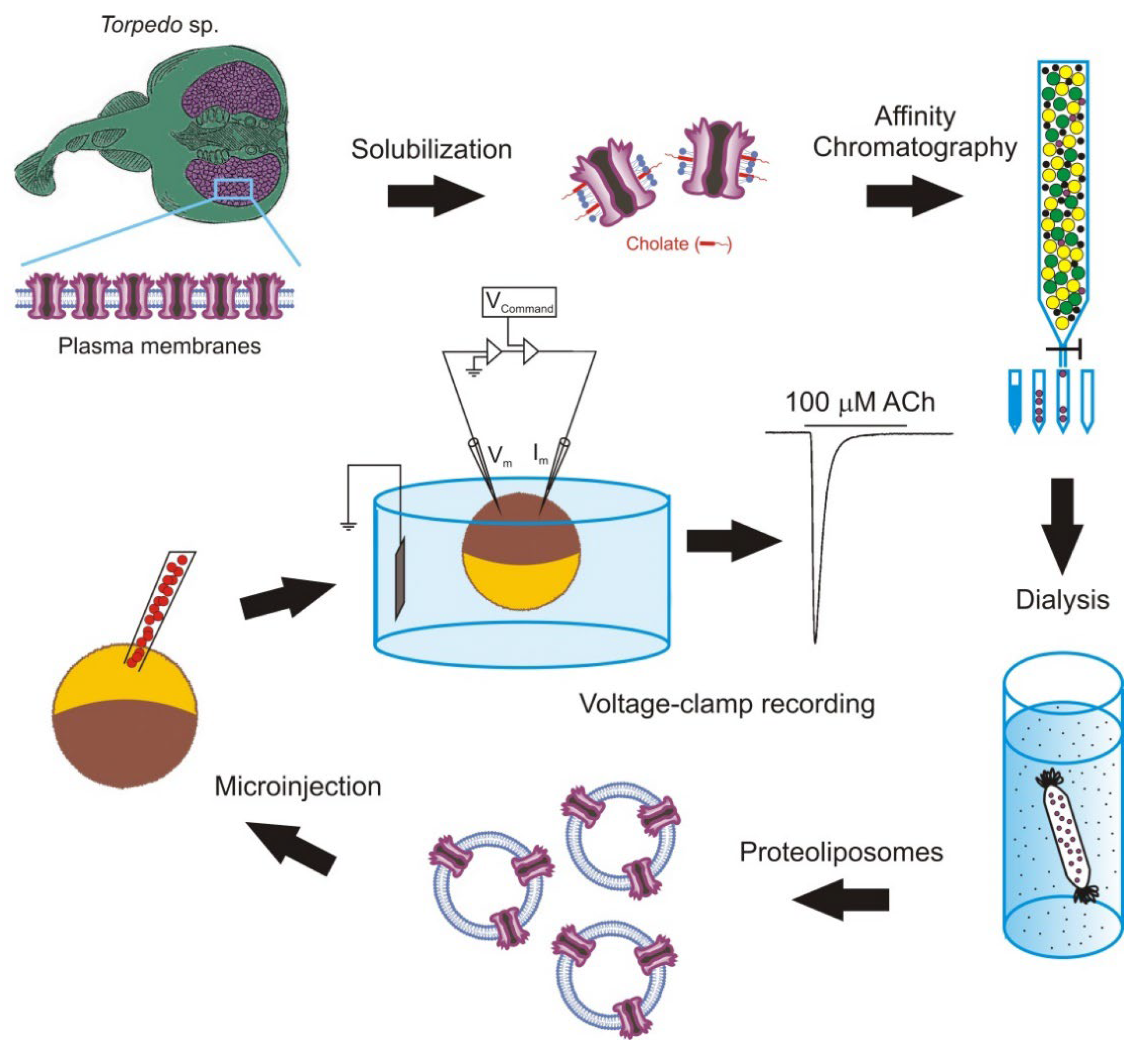
Figure 2. Methodology followed to microtransplant foreign membrane proteins to oocytes. Scheme of the steps to microtransplant the purified and reconstituted nicotinic acetylcholine (ACh) receptors (nAChRs) to the Xenopus oocyte membrane to carry out detailed functional studies.
It should be noted that the goal of this experimental approach is not to use oocytes as a factory to generate proteins, but as a convenient cellular system to carry out detailed functional studies of the transplanted membrane proteins. Nevertheless, the use of purified and reconstituted proteins, instead of fragments of cellular membranes, has several advantages including: (i) it allows for the study of single molecular entities; (ii) it does not require the transplanted protein to be highly expressed in the plasma membrane, although the presence of a large amount of protein simplifies its purification; and (iii) it makes it possible to study the influence that the lipid composition of the reconstitution matrix has on both the function of the transplanted protein and the process of fusion between the vesicular and cellular membranes. This later point is of special relevance since many proteins need to interact with specific lipids for developing their full functional activity [70][71]. Consequently, the microtransplantation of purified and reconstituted proteins into the Xenopus oocyte membrane arises as an excellent way to unravel the lipid–protein interactions, since it allows researchers to both insert proteins bound to specific lipids, which can even be labelled, and to selectively modify the lipid content of the cell membrane. Using this approach, it is possible to change not only the ratio of different phospholipids surrounding the protein to determine their functional relevance, but also the charge or the length of the acyl chains to induce local changes in the bilayer thickness and elasticity, which might also be important for the protein activity [72][73]. Thus, this approach constitutes a very useful extension to the classical use of cDNA or mRNA for the functional study of ion channels and neurotransmitter receptors.
This entry is adapted from the peer-reviewed paper 10.3390/membranes12100986
References
- Dumont, J.N. Oogenesis in Xenopus laevis (Daudin). I. Stages of oocyte development in laboratory maintained animals. J. Morphol. 1972, 136, 153–179.
- Miledi, R.; Parker, I.; Sumikawa, K. Transplanting receptors from brains into oocytes. In FIDIA Research Foundation Neuroscience Award Lectures; Raven Press, Ltd.: New York, NY, USA, 1989; Volume 3, pp. 57–90.
- Gurdon, J.B. Introductory comments: Xenopus as a laboratory animal. In The Biology of Xenopus; Tinsley, R.C., Kobel, H.R., Eds.; Clarendon Press: Oxford, UK, 1996; pp. 3–6.
- Mowry, K.L. Using the Xenopus Oocyte Toolbox. Cold Spring Harb. Protoc. 2020, 4, 095844.
- De Robertis, E.M.; Gurdon, J.B. A Brief History of Xenopus in Biology. Cold Spring Harb. Protoc. 2021, 12, 107615.
- Kusano, K.; Miledi, R.; Stinnakre, J. Acetylcholine receptors in the oocyte membrane. Nature 1977, 270, 739–741.
- Kusano, K.; Miledi, R.; Stinnakre, J. Cholinergic and catecholaminergic receptors in the Xenopus oocyte membrane. J. Physiol. 1982, 328, 143–170.
- Dascal, N. The use of Xenopus oocytes for the study of ion channels. Crit. Rev. Biochem. Mol. Biol. 1987, 22, 317–387.
- Sobczak, K.; Bangel-Ruland, N.; Leier, G.; Weber, W.M. Endogenous transport systems in the Xenopus laevis oocyte plasma membrane. Methods 2010, 51, 183–189.
- Martinez-Torres, A.; Pereida-Jaramillo, E. The use of Xenopus oocytes to study the biophysics and pharmacological properties of receptors and channels. In Xenopus: From Basic Biology to Disease Models in the Genomic Era; Fainsod, A., Moody, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2022; pp. 143–151.
- Weber, W.M. Ion currents of Xenopus laevis oocytes: State of the art. Biochim. Biophys. Acta 1999, 1421, 213–233.
- Eusebi, F.; Palma, E.; Amici, M.; Miledi, R. Microtransplantation of ligand-gated receptor-channels from fresh or frozen nervous tissue into Xenopus oocytes: A potent tool for expanding functional information. Prog. Neurobiol. 2009, 88, 32–40.
- Zeng, S.L.; Sudlow, L.C.; Berezin, M.Y. Using Xenopus oocytes in neurological disease drug discovery. Expert Opin. Drug Discov. 2020, 15, 39–52.
- Stühmer, W.; Parekh, A.B. Electrophysiological recordings from Xenopus oocytes. In Single-Channel Recording; Sakmann, B., Neher, E., Eds.; Springer: Boston, MA, USA, 1995; pp. 341–356.
- Palma, E.; Trettel, F.; Fucile, S.; Renzi, M.; Miledi, R.; Eusebi, F. Microtransplantation of membranes from cultured cells to Xenopus oocytes: A method to study neurotransmitter receptors embedded in native lipids. Proc. Natl. Acad. Sci. USA 2003, 100, 2896–2900.
- Kvist, T.; Hansen, K.B.; Bräuner-Osborne, H. The use of Xenopus oocytes in drug screening. Expert Opin. Drug Discov. 2011, 6, 141–153.
- Gurdon, J.B.; Wickens, M.P. The use of Xenopus oocytes for the expression of cloned genes. Methods Enzym. 1983, 101, 370–386.
- Krogh, A. The progress of Physiology. Science 1929, 70, 200–204.
- Demuro, A.; Parker, I. Optical single-channel recording: Imaging Ca2+ flux through individual N-type voltage-gated channels expressed in Xenopus oocytes. Cell Calcium 2003, 34, 499–509.
- Gurdon, J.B.; Lane, C.D.; Woodland, H.R.; Marbaix, G. Use of frog eggs and oocytes for the study of messenger RNA and its translation in living cells. Nature 1971, 233, 177–182.
- Barnard, E.A.; Miledi, R.; Sumikawa, K. Translation of exogenous messenger RNA coding for nicotinic acetylcholine receptors produces functional receptors in Xenopus oocytes. Proc. R. Soc. Lond. Ser. B. Biol. Sci. 1982, 215, 241–246.
- Miledi, R.; Parker, I.; Sumikawa, K. Properties of acetylcholine receptors translated by cat muscle mRNA in Xenopus oocytes. EMBO J. 1982, 1, 1307–1312.
- Noda, M.; Ikeda, T.; Suzuki, H.; Takeshima, H.; Takahashi, T.; Kuno, M.; Numa, S. Expression of functional sodium channels from cloned cDNA. Nature 1986, 322, 826–828.
- Soreq, H.; Seidman, S. Xenopus oocyte microinjection: From gene to protein. Methods Enzym. 1992, 207, 225–265.
- Miller, A.J.; Zhou, J.J. Xenopus oocytes as an expression system for plant transporters. Biochim. Biophys. Acta 2000, 1465, 343–358.
- Miledi, R.; Palma, E.; Eusebi, F. Microtransplantation of neurotransmitter receptors from cells to Xenopus oocyte membranes: New procedure for ion channel studies. Methods Mol. Biol. 2006, 322, 347–355.
- Terhag, J.; Cavara, N.A.; Hollmann, M. Cave Canalem: How endogenous ion channels may interfere with heterologous expression in Xenopus oocytes. Methods 2010, 51, 66–74.
- Zhang, R.B.; Logee, K.A.; Verkman, A.S. Expression of mRNA coding for kidney and red cell water channels in Xenopus oocytes. J. Biol. Chem. 1990, 265, 15375–15378.
- Preston, G.M.; Carroll, T.P.; Guggino, W.B.; Agre, P. Appearance of water channels in Xenopus oocytes expressing red cell CHIP28 protein. Science 1992, 256, 385–387.
- Sadler, S.E.; Maller, J.L. Progesterone inhibits adenylate cyclase in Xenopus oocytes. Action on the guanine nucleotide regulatory protein. J. Biol. Chem. 1981, 256, 6368–6373.
- Aleu, J.; Ivorra, I.; Lejarreta, M.; González-Ros, J.M.; Morales, A.; Ferragut, J.A. Functional incorporation of P-glycoprotein into Xenopus oocyte plasma membrane fails to elicit a swelling-evoked conductance. Biochem. Biophys. Res. Commun. 1997, 237, 407–412.
- Leduc-Nadeau, A.; Lahjouji, K.; Bissonnette, P.; Lapointe, J.Y.; Bichet, D.G. Elaboration of a novel technique for purification of plasma membranes from Xenopus laevis oocytes. Am. J. Physiol. Cell Physiol. 2007, 292, C1132–C1136.
- Ivorra, I.; Fernández, A.; Gal, B.; Aleu, J.; González-Ros, J.M.; Ferragut, J.A.; Morales, A. Protein orientation affects the efficiency of functional protein transplantation into the Xenopus oocyte membrane. J. Membr. Biol. 2002, 185, 117–127.
- Murenzi, E.; Toltin, A.C.; Symington, S.B.; Morgan, M.M.; Clark, J.M. Evaluation of microtransplantation of rat brain neurolemma into Xenopus laevis oocytes as a technique to study the effect of neurotoxicants on endogenous voltage-sensitive ion channels. Neurotoxicology 2017, 60, 260–273.
- Miledi, R. A calcium-dependent transient outward current in Xenopus laevis oocytes. Proc. R. Soc. Lond. B. Biol. Sci. 1982, 215, 491–497.
- Barish, M.E. A transient calcium-dependent chloride current in the immature Xenopus oocyte. J. Physiol. 1983, 342, 309–325.
- Browne, C.L.; Wiley, H.S.; Dumont, J.N. Oocyte-follicle cell gap junctions in Xenopus laevis and the effects of gonadotropin on their permeability. Science 1979, 203, 182–183.
- Zhang, J.; Yuan, H.; Yao, X.; Chen, S. Endogenous ion channels expressed in human embryonic kidney (HEK-293) cells. Pflugers Arch.-Eur. J. Physiol. 2022, 474, 665–680.
- Madeja, M.; Musshoff, U.; Speckmann, E.J. Follicular tissues reduce drug effects on ion channels in oocytes of Xenopus laevis. Eur. J. Neurosci. 1997, 9, 599–604.
- Gielen, M.; Corringer, P.-J. The dual-gate model for pentameric ligand-gated ion channels activation and desensitization. J. Physiol. 2018, 596, 1873–1902.
- Buller, A.L.; White, M.M. Altered patterns of N-linked glycosylation of the Torpedo acetylcholine receptor expressed in Xenopus oocytes. J. Membr. Biol. 1990, 115, 179–189.
- Sivilotti, L.G.; McNeil, D.K.; Lewis, T.M.; Nassar, M.A.; Schoepfer, R.; Colquhoun, D. Recombinant nicotinic receptors, expressed in Xenopus oocytes, do not resemble native rat sympathetic ganglion receptors in single-channel behaviour. J. Physiol. 1997, 500, 123–138.
- Truong, A.; Xing, X.; Forsayeth, J.R.; Dwoskin, L.P.; Crooks, P.A.; Cohen, B.N. Pharmacological differences between immunoisolated native brain and heterologously expressed rat alpha4beta2 nicotinic receptors. Brain Res. Mol. Brain Res. 2001, 96, 68–76.
- Jospin, M.; Bonneau, B.; Lainé, V.; Bessereau, J.L. An extracellular scaffolding complex confers unusual rectification upon an ionotropic acetylcholine receptor in C. elegans. Proc. Natl. Acad. Sci. USA 2022, 119, e2113545119.
- Opekarová, M.; Tanner, W. Specific lipid requirements of membrane proteins-a putative bottleneck in heterologous expression. Biochim. Biophys. Acta 2003, 1610, 11–22.
- Mesoy, S.M.; Bridgland-Taylor, M.; Lummis, S.C.R. Mutations of the nACh Receptor M4 Helix Reveal Different Phenotypes in Different Expression Systems: Could Lipids be Responsible? Front. Physiol. 2022, 13, 850782.
- Marsal, J.; Tigyi, G.; Miledi, R. Incorporation of acetylcholine receptors and Cl- channels in Xenopus oocytes injected with Torpedo electroplaque membranes. Proc. Natl. Acad. Sci. USA 1995, 92, 5224–5228.
- Bernareggi, A.; Reyes-Ruiz, J.M.; Lorenzon, P.; Ruzzier, F.; Miledi, R. Microtransplantation of acetylcholine receptors from normal or denervated rat skeletal muscles to frog oocytes. J. Physiol. 2011, 589, 1133–1142.
- Palma, E.; Inghilleri, M.; Conti, L.; Deflorio, C.; Frasca, V.; Manteca, A.; Pichiorri, F.; Roseti, C.; Torchia, G.; Limatola, C.; et al. Physiological characterization of human muscle acetylcholine receptors from ALS patients. Proc. Natl. Acad. Sci. USA 2011, 108, 20184–20188.
- Olivera-Bravo, S.; Ivorra, I.; Morales, A. Differential effects of quaternary ammonium anticholinesterases on microtransplanted neuroreceptors: Selective modulation of nicotinic receptor function. J. Mol. Neurosci. 2010, 40, 251–252.
- Sanna, E.; Motzo, C.; Usala, M.; Pau, D.; Cagetti, E.; Biggio, G. Functional changes in rat nigral GABA(A) receptors induced by degeneration of the striatonigral GABAergic pathway: An electrophysiological study of receptors incorporated into Xenopus oocytes. J. Neurochem. 1998, 70, 2539–2544.
- Miledi, R.; Eusebi, F.; Martínez-Torres, A.; Palma, E.; Trettel, F. Expression of functional neurotransmitter receptors in Xenopus oocytes after injection of human brain membranes. Proc. Natl. Acad. Sci. USA 2002, 99, 13238–13242.
- Palma, E.; Torchia, G.; Limatola, C.; Trettel, F.; Arcella, A.; Cantore, G.; Di Gennaro, G.; Manfredi, M.; Esposito, V.; Quarato, P.P.; et al. BDNF modulates GABAA receptors microtransplanted from the human epileptic brain to Xenopus oocytes. Proc. Natl. Acad. Sci. USA 2005, 102, 1667–1672.
- Palma, E.; Spinelli, G.; Torchia, G.; Martínez-Torres, A.; Ragozzino, D.; Miledi, R.; Eusebi, F. Abnormal GABAA receptors from the human epileptic hippocampal subiculum microtransplanted to Xenopus oocytes. Proc. Natl. Acad. Sci. USA. 2005, 102, 2514–2518.
- Palma, E.; Amici, M.; Sobrero, F.; Spinelli, G.; Di Angelantonio, S.; Ragozzino, D.; Mascia, A.; Scoppetta, C.; Esposito, V.; Miledi, R.; et al. Anomalous levels of Cl- transporters in the hippocampal subiculum from temporal lobe epilepsy patients make GABA excitatory. Proc. Natl. Acad. Sci. USA 2006, 103, 8465–8468.
- Palma, E.; Roseti, C.; Maiolino, F.; Fucile, S.; Martinello, K.; Mazzuferi, M.; Aronica, E.; Manfredi, M.; Esposito, V.; Cantore, G.; et al. GABA(A)-current rundown of temporal lobe epilepsy is associated with repetitive activation of GABA(A) “phasic” receptors. Proc. Natl. Acad. Sci. USA 2007, 104, 20944–20948.
- Palma, E.; Ragozzino, D.; Di Angelantonio, S.; Mascia, A.; Maiolino, F.; Manfredi, M.; Cantore, G.; Esposito, V.; Di Gennaro, G.; Quarato, P.; et al. The antiepileptic drug levetiracetam stabilizes the human epileptic GABAA receptors upon repetitive activation. Epilepsia 2007, 48, 1842–1849.
- Limon, A.; Reyes-Ruiz, J.M.; Miledi, R. Microtransplantation of neurotransmitter receptors from postmortem autistic brains to Xenopus oocytes. Proc. Natl. Acad. Sci. USA 2008, 105, 10973–10977.
- Alberola-Die, A.; Fernández-Ballester, G.; González-Ros, J.M.; Ivorra, I.; Morales, A. Muscle-type nicotinic receptor blockade by diethylamine, the hydrophilic moiety of lidocaine. Front. Mol. Neurosci. 2016, 9, 12.
- Alberola-Die, A.; Fernández-Ballester, G.; González-Ros, J.M.; Ivorra, I.; Morales, A. Muscle-type nicotinic receptor modulation by 2,6-dimethylaniline, a molecule resembling the hydrophobic moiety of lidocaine. Front. Mol. Neurosci. 2016, 9, 127.
- Mazzo, F.; Zwart, R.; Serratto, G.M.; Gardinier, K.M.; Porter, W.; Reel, J.; Maraula, G.; Sher, E. Reconstitution of synaptic ion channels from rodent and human brain in Xenopus oocytes: A biochemical and electrophysiological characterization. J. Neurochem. 2016, 138, 384–396.
- Burgos, J.S.; Aleu, J.; Barat, A.; Solsona, C.; Marsal, J.; Ramírez, G. Kainate-triggered currents in Xenopus oocytes injected with chick retinal membrane fragments: Effect of guanine nucleotides. Investig. Ophthalmol. Vis. Sci. 2003, 44, 3124–3129.
- Bernareggi, A.; Dueñas, Z.; Reyes-Ruiz, J.M.; Ruzzier, F.; Miledi, R. Properties of glutamate receptors of Alzheimer’s disease brain transplanted to frog oocytes. Proc. Natl. Acad. Sci. USA 2007, 104, 2956–2960.
- Sandoval, M.; Sandoval, R.; Thomas, U.; Spilker, C.; Smalla, K.H.; Falcon, R.; Marengo, J.J.; Calderón, R.; Saavedra, V.; Heumann, R.; et al. Antagonistic effects of TrkB and p75(NTR) on NMDA receptor currents in post-synaptic densities transplanted into Xenopus oocytes. J. Neurochem. 2007, 101, 1672–1684.
- Miledi, R.; Dueñas, Z.; Martinez-Torres, A.; Kawas, C.H.; Eusebi, F. Microtransplantation of functional receptors and channels from the Alzheimer’s brain to frog oocytes. Proc. Natl. Acad. Sci. USA 2004, 101, 1760–1763.
- Ivorra, I.; Henriquez, M.; Lax, P.; Riquelme, G.; Morales, A. Functional transplantation of chloride channels from the human syncytiotrophoblast microvillous membrane to Xenopus oocytes. Pflug. Arch.-Eur. J. Physiol. 2002, 444, 685–691.
- Morales, A.; Aleu, J.; Ivorra, I.; Ferragut, J.A.; González-Ros, J.M.; Miledi, R. Incorporation of reconstituted acetylcholine receptors from Torpedo into the Xenopus oocyte membrane. Proc. Natl. Acad. Sci. USA 1995, 92, 8468–8472.
- Morales, A.; de Juan, E.; Fernández-Carvajal, A.M.; Martinez-Pinna, J.; Poveda, J.A.; Encinar, J.A.; Ivorra, I.; González-Ros, J.M. Nicotinic acetylcholine receptor properties are modulated by surrounding lipids: An in vivo study. J. Mol. Neurosci. 2006, 30, 5–6.
- Le Cahérec, F.; Bron, P.; Verbavatz, J.M.; Garret, A.; Morel, G.; Cavalier, A.; Bonnec, G.; Thomas, D.; Gouranton, J.; Hubert, J.F. Incorporation of proteins into (Xenopus) oocytes by proteoliposome microinjection: Functional characterization of a novel aquaporin. J. Cell Sci. 1996, 109, 1285–1295.
- Thompson, M.J.; Baenziger, J.E. Ion channels as lipid sensors: From structures to mechanisms. Nat. Chem. Biol. 2020, 16, 1331–1342.
- Vallés, A.S.; Barrantes, F.J. Dysregulation of neuronal nicotinic acetylcholine receptor-cholesterol crosstalk in autism spectrum disorder. Front. Mol. Neurosci. 2021, 14, 744597.
- Martinac, B.; Hamill, O.P. Gramicidin A channels switch between stretch activation and stretch inactivation depending on bilayer thickness. Proc. Natl. Acad. Sci. USA 2002, 99, 4308–4312.
- Lundbaek, J.A.; Birn, P.; Hansen, A.J.; Søgaard, R.; Nielsen, C.; Girshman, J.; Bruno, M.J.; Tape, S.E.; Egebjerg, J.; Greathouse, D.V.; et al. Regulation of sodium channel function by bilayer elasticity: The importance of hydrophobic coupling. Effects of Micelle-forming amphiphiles and cholesterol. J. Gen. Physiol. 2004, 123, 599–621.
This entry is offline, you can click here to edit this entry!
