You're using an outdated browser. Please upgrade to a modern browser for the best experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Reinforced cement concrete (RCC) is a versatile material that deteriorates over time due to corrosion when exposed to any surrounding aggressive environment. In order to avoid this ramification, many researchers have carried out different works to find the most feasible way to reduce corrosion. To tackle this at the initial stage itself, one such convenient method is through the application of inhibitors. These inhibitors have the most applications in their use as an admixture.
- corrosion
- reinforced cement concrete
- green corrosion inhibitor
1. Inhibitors Definition
Inhibitors are substances that are added in parts per million concentrations to reduce or prevent steel rebar corrosion. The level of inhibition is based on the nature of the metallic specimen to be adopted and the type of exposure medium[1]. They reduce the corrosion action on the vulnerable material by performing as a barrier, or by materialising an adsorbed film or by decelerating the cathodic or anodic processes, or a combination of both, thus improving the durability of RCC structures[2][3]. Therefore, the application of inhibitors in reinforced concrete structure is an effective approach in regulating the corrosion to reasonable levels [4]. It is not sufficient to perform only the anticorrosion tests in order to study the binding strength of reinforced concrete and inhibitor, but also requires various mechanical tests which needs to be performed [5]. Inhibitors are typically admixed in low permeability concrete specimens[6]. An effective corrosion inhibitor is the one which shows better adsorption potential on the metal surface, thus providing a tight adherent coating film which can be accomplished either through the process of physisorption or chemisorption, or by both [7][8]. These inhibitors also have their application in RCC as shown below in Figure 1 [9].
Figure 1. Inhibitors Application to Reinforced Cement Concrete.
Factors affecting the choice of suitable Inhibitor selection:
-
The form of chemicals used in the industry;
-
Concentration of the inhibitor molecules;
-
Category of metals to be adopted;
-
Temperature.
Factors affecting the inhibitors adsorption capacity [10]:
-
Characteristics, properties, composition and reaction of metal surface;
-
Chemical structure of inhibitor; and
-
Kind of aggressive mediums.
Properties of corrosion Inhibitors[7]:
-
Significant adsorption on the metal surface;
-
Altering the anodic or cathodic reactions or both;
-
Reducing the dispersion rate of reactants on the steel surface; and
-
Lowering the electrical resistance of metal surface.
It is highly necessary to maintain an appropriate level of inhibitor concentration on the metallic surface in order to cover the metal completely, otherwise there will be localised corrosion at the unprotected sites[11]. The classification of corrosion inhibitors can be seen below in Figure 2.

Figure 2. Classification of Corrosion Inhibitors.
In order to mitigate corrosion in new concrete structures, inorganic inhibitors are added to the mixing water at the initial stage. Sodium nitrite, calcium nitrite, and other nitrites were the most well-known and sought-after inorganic-based corrosion inhibitors in the previous century. Calcium Nitrite was shown to be the most effective RCC corrosion inhibitor when compared to its alternatives.
Even with their effectiveness, the bulk of the inorganic corrosion inhibitors such as nitrites, tungstate, benzoate, molybdates phosphates, chromate and silicates, continue to be banned around the world on account of their high biological toxicity and carcinogenicity. One such toxic inhibitor is nitrites which have been found to delay the setting time, and lowering the strength of concrete thus affecting the overall performance of the structure. Similarly, chromate anti corrosive properties, although beneficial, are limited as they are shown to have water polluting and carcinogenic properties. Overall, inorganic corrosion inhibitors have been shown to be non-biodegradable, noxious, uneconomical along with damaging multiple human organs such as liver and kidneys due to prolonged exposure[12].
Organic Inhibitors are unusually the effective mixed class of inhibitors which exhibits both anodic and cathodic operations in a simultaneous manner because of the existence of hetero atoms such as Oxygen, Nitrogen, Sulphur, Phosphorous, conjugated double bonds [13] and aromatic rings in it. These hetero atoms perform as an active core material for the physical and chemical adsorption process to occur on the surface of steel, possessing a high electron density[14]. Through the transport of lone pair of electrons from the atoms of the inhibitor to the stable metal surface, these molecules present in the inhibitor form a stronger link with the steel concrete interface, thus obstructing the active corrosion site [15], hence preventing the entry of destructive species which are accountable for the depassivation of rebar [14] such as CO2, SO4−, Cl−, and moisture content on the surface of steel [16].
Some of other parameters influencing the performance of corrosion inhibitors are:
-
Chemical composition of functional groups;
-
The existence of pi-bonds;
-
The electronic characteristics of the molecule; and
-
Non-bonding p-orbitals [17].
The mechanism of organic corrosion inhibitor relies on its chemical constituents. When these inhibitors are admixed to a solution, they are adsorbed strongly on the metal’s surface and embedded in concrete, thus displacing water molecules and destructive agents such as chloride ions from its surface. The drawbacks of both organic and hybrid inhibitors are detailed below in Figure 3.
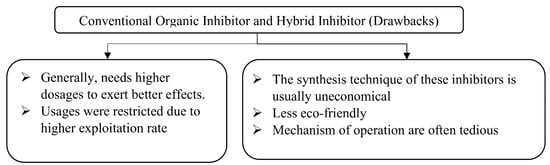
Figure 3. Drawbacks of Conventional Organic Inhibitor and Hybrid Inhibitors.
2. Green Corrosion Inhibitors
Human life expectancy and environmental consciousness have both substantially developed along with the advancement of society[18]. The majority of corrosion inhibitors used by several researchers over the years are synthetic chemicals which are found to be costly and harmful to the environment. Accordingly, there is a requirement for an inhibitor which can be highly protective to the environment. As a result, there has been an increasing demand in recent years for the hunt for inhibitors which are proven to be economical [17], less harmful and biodegradable when compared with the conventional inhibitors. Thus, the environmentally friendly corrosion inhibitors are gaining a lot of popularity from researchers all over the globe [19]. During this stage, scientists started to target completely the “green” botanic corrosion inhibitors leading to the increased demand in the field of Science and Technology [20]. Recently, various research has been carried out by several authors using the green corrosion inhibitor as an admixture in RCC to test its efficiency experimentally in order to increase the mean service life of structures. The efficiency of inhibitor is based on the characteristics of its chemical ingredients [16]. It also showed an improved consistency in producing the results. Owing to the accessibility and much better inhibitive characteristics, the green corrosion inhibitors can be taken as an effective alternative to the prevalent conventional organic inhibitors. These are the compounds which are going to be used more prominently in the upcoming years [21]. The two subcategories of green corrosion inhibitors are classified in Figure 4.

Figure 4. Classification of green corrosion inhibitors.
Inorganic green inhibitor initiates the formation of adsorption layers that tend to be brittle, thereby revealing the surface of the metal vulnerable to pitting and crevice corrosion attacks. In contrast, organic green inhibitor, when weighed upon with inorganic corrosion inhibitors, tend to passivate the surface of the metal consistently and yield the maximum possible protection against the destructive medium [21].
There are many varieties of green corrosion inhibitors that are gaining traction such as biopolymers, surface active agent, pharmaceutical compounds, chitosan, honey, yeast, plant extracts, and amino acids, etc., to reduce the corrosion in reinforced concrete structures because of their easy availability, eco-friendly, biodegradability, low toxicity, and renewable properties. When these eco based inhibitors are added as an admixture to the mixing water in the reinforced concrete specimen, the organic compounds present in these inhibitor sources are adsorbed on the surface of metal, forming a passivation layer that protects the active area from the action of corrosion [17][19]. Figure 5 shows the various types of green corrosion inhibitors that are obtainable.
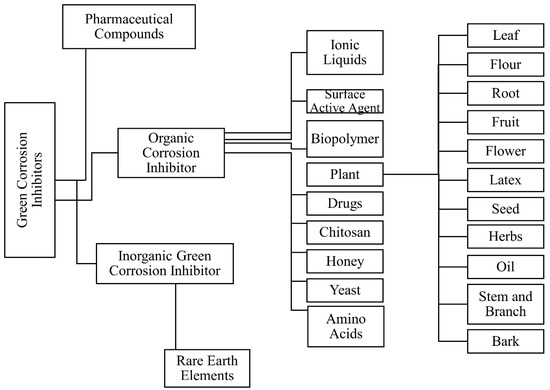
Figure 5. Flowchart of various Green Corrosion Inhibitors.
It is exceedingly practical and affordable to obtain or collect the ingredients that are necessary for inhibition from natural plants along with their waste materials due to the presence of phytochemicals which are able to strongly bind on the surface of metal. The extraction of green corrosion inhibitor from natural plants has become a popular subject in the anticorrosion industry. Owing to the accessibility and much better inhibitive characteristics, the green corrosion inhibitors can be adopted as an effective alternative to the prevalent conventional organic inhibitors [15].
In order to hinder scale development and microbial corrosion, plant substances have the ability to remove oxygen as well as several types of reactive oxygen from a biological environment. The occurrence of heterocyclic compounds such as sulphur, oxygen, or nitrogen atoms, aromatic rings, pi-electrons and specifically its polar functionalities all aid to this performance. The reaction centre that initiates the adsorption method is commonly represented by the polar function [22]. Plant extracts can be classified as green and sustainable materials to be used as eco inhibitors for metals and alloy mixtures in aggressive mediums such as HCl, H2SO4, H3PO4, and HNO3 due to their diverse natural origins, as well as their environmental-friendly isolation[20].
Through the action of photosynthesis, plants transform radiation energy absorbed from sunlight into useful organic compounds such as carbohydrates. These plants are the natural producers of the following raw materials namely fruits, vegetables, oils, woods, and dyes which have a wide variety of regular use in people's daily lives. Through the phenomenon of photosynthesis, plants possess an ability to absorb the following contaminants such as the harmful CO2 gas, toxic minerals, etc., hence purifying the area around it[20].
The green inhibitor components are obtained from their respective plant sources primarily through the process of extraction. This is a tedious process due to the presence of different polarities of the chemical compounds from the same plant source. The nature of the extraction solvent to that of the organic compound is the most significant factor that might influence the extraction outcome [22]. The plant extraction parameters that need to be noted is listed in Figure 6.
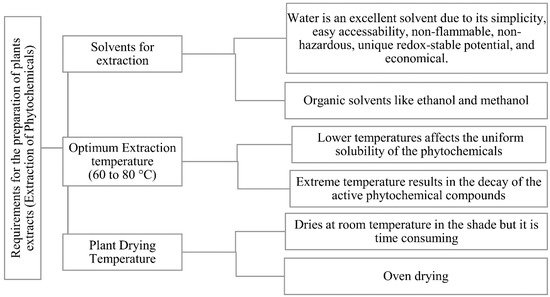
Figure 6. Parameters considered during the preparation of plant extract [20].
To recognise the distinctive property of water retention, plant extracts such as tannins, sugars and starch are employed as curing members in the form of bio admixtures added to concrete as they impact its characteristics in a positive manner[23][24].
This entry is adapted from the peer-reviewed paper 10.3390/buildings12101682
References
- Zora Pilić; Ivan Dragičević; Ivana Martinović; The anti-corrosion behaviour of Satureja montana L. extract on iron in NaCl solution. Open Chemistry 2019, 17, 1087-1094, 10.1515/chem-2019-0126.
- Nahida Nisar; Javed Ahmed Bhat; Effect of Coupled Deterioration Mechanisms on Corrosion of Steel Reinforcement: The Role of Chloride Ion Ingress, Freeze–Thaw Cycles and Green Corrosion Inhibitor. Journal of Bio- and Tribo-Corrosion 2021, 7, 1-9, 10.1007/s40735-021-00564-x.
- Karthick Subbiah, Park TaeJoon, Lee Han Seung; Corrosion Inhibition Properties of Conifer Cone (Pinus resinosa) Extract in Chloride Contaminated Concrete Pore Solutions.. The Korean Institute of Building Construction 2021, 1, 63-64, https://koreascience.kr/article/CFKO202121751304268.page.
- N. Delbianco, C. Priano, M. Pérez, N. F. Ortega; Plant extracts as corrosion inhibitors: Effect of lyophilization. Thinking 2020, 15, 16, http://laccei.org/LACCEI2020-VirtualEdition/student_papers/SP659.pdf.
- Joshua Olusegun Okeniyi; Abimbola Patricia Idowu Popoola; Cleophas Akintoye Loto; Corrosion-inhibition and compressive-strength performance of Phyllanthus muellerianus and triethanolamine on steel-reinforced concrete immersed in saline/marine simulating-environment. Energy Procedia 2017, 119, 972-979, 10.1016/j.egypro.2017.07.130.
- Seyedmojtaba Ghoreishiamiri; Pandian Bothi Raja; Mohammad Ismail; Seyedeh Faezeh Hashemi Karouei; Parham Forouzani; Areca Catechu: An Eco-Friendly Corrosion Inhibitor for Reinforced Concrete Structures in Corrosive Mediums. Journal of Bio- and Tribo-Corrosion 2021, 7, 1-5, 10.1007/s40735-020-00464-6.
- Anjali Peter; Ime Bassey Obot; Sanjay K. Sharma; Use of natural gums as green corrosion inhibitors: an overview. International Journal of Industrial Chemistry 2015, 6, 153-164, 10.1007/s40090-015-0040-1.
- Ahmed A. ElShami; Stéphanie Bonnet; Mohamed Hussein Makhlouf; A. Khelidj; N. Leklou; Novel green plants extract as corrosion inhibiting coating for steel embedded in concrete. Pigment & Resin Technology 2020, 49, 501-514, 10.1108/prt-09-2019-0078.
- Kelechi Okwulehie, Kpegara Saana N, Charles Kennedy; Electrochemical Techniques Assessment of Chloride Threshold for Reinforcing Steel Corrosion in Concrete Structures. Saudi Journal of Engineering and Technology 2021, 6, 290-306, 10.36348/sjet.2021.v06i08.007.
- S. A. Abo El-Enin, Ashraf Amin; Review of corrosion inhibitors for industrial applications. International Journal of Engineering Research and Reviews 2015, 3, 127-145, ISSN 2348-697X.
- Jainam Panchal; Dhruval Shah; Rudra Patel; Shrey Shah; Madhav Prajapati; Manan Shah; Comprehensive Review and Critical Data Analysis on Corrosion and Emphasizing on Green Eco-friendly Corrosion Inhibitors for Oil and Gas Industries. Journal of Bio- and Tribo-Corrosion 2021, 7, 1-29, 10.1007/s40735-021-00540-5.
- Mohammad Ali Asaad; Mohammad Ismail; Mahmood Tahir; Ghasan Fahim Huseien; Pandian Bothi Raja; Yuli Panca Asmara; Enhanced corrosion resistance of reinforced concrete: Role of emerging eco-friendly Elaeis guineensis/silver nanoparticles inhibitor. Construction and Building Materials 2018, 188, 555-568, 10.1016/j.conbuildmat.2018.08.140.
- Seyedmojtaba Ghoreishiamiri; Pandian Bothi Raja; Mohammad Ismail; Seyedeh Faezeh Hashemi Karouei; Properties of Contaminated Reinforced Concrete Added by Areca catechu Leaf Extract as an Eco-friendly Corrosion Inhibitor. Journal of Bio- and Tribo-Corrosion 2020, 6, 1-14, 10.1007/s40735-020-00430-2.
- Mohammad Ali Asaad; Commercial and Green Corrosion Inhibitors for Reinforced Concrete Structures: A Review. International Journal of Current Science Research and Review 2021, 4, 88-89, 10.47191/ijcsrr/v4-i2-04.
- Amir Nobahar; Jorge Dias Carlier; Maria Graça Miguel; Maria Clara Costa; A review of plant metabolites with metal interaction capacity: a green approach for industrial applications. BioMetals 2021, 34, 761-793, 10.1007/s10534-021-00315-y.
- Yuli Panca Asmara; Tedi Kurniawan; Agus Geter Edy Sutjipto; Jamiluddin Jafar; Application of Plants Extracts as Green Corrosion Inhibitors for Steel in Concrete - A review. Indonesian Journal of Science and Technology 2018, 3, 158-170, 10.17509/ijost.v3i2.12760.
- M. F. Montemor; Fostering Green Inhibitors for Corrosion Prevention. Active Protective Coatings 2016, 233, 107-137, 10.1007/978-94-017-7540-3_6.
- Tejada Tovar, C. N., Barrios Fontalvo, M., Villabona Ortíz, A., Castillo Mercado, F., & Ramírez Arenilla, B.; . Evaluation of Cedrela odorata Linnaeus extract in concrete handling and resistance to compression.. EIA 2021, 18, 36003, https://doi.org/10.24050/reia.v18i36.1497.
- Jitendra Kumar Singh; Hyun-Min Yang; Han-Seung Lee; Soumen Mandal; Fahid Aslam; Rayed Alyousef; Role of L-arginine on the formation and breakdown of passive film onto the steel rebars surface in chloride contaminated concrete pore solution. Journal of Molecular Liquids 2021, 337, 116454, 10.1016/j.molliq.2021.116454.
- Chandrabhan Verma; Eno E. Ebenso; Indra Bahadur; M.A. Quraishi; An overview on plant extracts as environmental sustainable and green corrosion inhibitors for metals and alloys in aggressive corrosive media. Journal of Molecular Liquids 2018, 266, 577-590, 10.1016/j.molliq.2018.06.110.
- Siti Zuliana Salleh; Abdul Hafidz Yusoff; Siti Koriah Zakaria; Mustaffa Ali Azhar Taib; Anasyida Abu Seman; Mohamad Najmi Masri; Mardawani Mohamad; Sarizam Mamat; Sharizal Ahmad Sobri; Arlina Ali; et al. Plant extracts as green corrosion inhibitor for ferrous metal alloys: A review. Journal of Cleaner Production 2021, 304, 127030, 10.1016/j.jclepro.2021.127030.
- Marwa Ben Harb; Samar Abubshait; Naceur Etteyeb; Madiha Kamoun; Adnene Dhouib; Olive leaf extract as a green corrosion inhibitor of reinforced concrete contaminated with seawater. Arabian Journal of Chemistry 2020, 13, 4846-4856, 10.1016/j.arabjc.2020.01.016.
- Muhammad Riaz Ahmad; Bing Chen; Syed Farasat Ali Shah; Mechanical and microstructural characterization of bio-concrete prepared with optimized alternative green binders. Construction and Building Materials 2021, 281, 122533, 10.1016/j.conbuildmat.2021.122533.
- Ramalingam Malathy; Ill-Min Chung; Mayakrishnan Prabakaran; Characteristics of fly ash based concrete prepared with bio admixtures as internal curing agents. Construction and Building Materials 2020, 262, 120596, 10.1016/j.conbuildmat.2020.120596.
This entry is offline, you can click here to edit this entry!
