Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Nutrition & Dietetics
Kynurenic acid (KYNA), a metabolite of tryptophan, is an endogenous substance produced intracellularly by various human cells. In addition, KYNA can be synthesized by the gut microbiome and delivered in food.
- food ingredients
- infant formula
- kynurenic acid
- nutrition
1. Kynurenic Acid in Food
1.1. Human Food
It has been repeatedly shown that KYNA is a natural component of food. Its content in food and food products varies within a wide range of concentrations, from trace amounts up to 2 mg/gram of chestnut honey [73,74,75,76,77,78,79,80] (Figure 3).
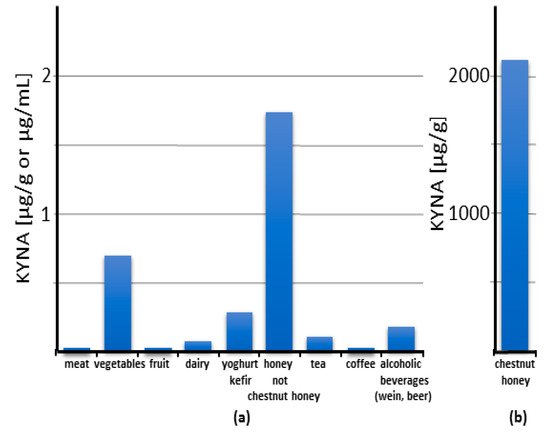
Figure 3. Content of kynurenic acid (KYNA) in food categories: an overview. The columns reflect the value of the highest reported content of KYNA in each category. Note that the scale on panel (b) is 1000 times larger than on panel (a).
1.1.1. Meat
It can be stated that the content of KYNA in meat is low: 0.0014, 0.0031, and 0.0037 μg/g wet weight in beef, pork, and fish, respectively. Even pig liver contains only slightly more KYNA—0.0091 μg/g wet weight. Since KYNA is rapidly excreted from the animal’s body (see Chapter 6), it does not seem possible to easily increase its amount in meat.
1.1.2. Vegetables
Vegetables are a richer source of KYNA (Figure 3). Cauliflower, potato, and broccoli are some of the richest sources, containing KYNA in amounts of 0.0473, 0.1301, and 0.4184 μg/g wet weight, respectively [73]. However, it should be noted that large differences between varieties exist. The comparison of 16 different edible potato varieties grown under similar soil and climatic conditions showed up to 10-fold differences in KYNA content, from 0.04 to 0.65 μg/g wet weight [76]. Similarly, the comparison of yellow- and purple-fleshed potato cultivar Ismena and Provita revealed a threefold difference in KYNA content, 0.226 and 0.683 μg/g wet weight, respectively [81]. The origin of KYNA in plants is poorly understood. Both its synthesis from kynurenine and the absorption of KYNA from the soil were presented [82]. Since KYNA is found in the soil in varying amounts, and in extraordinary large amounts in manure [82], its content in the plant may depend on the site and cultivation method. This may also be the reason for significant differences in KYNA content even in the same type of vegetable. At the same time, it provides an opportunity to increase KYNA content in the plant by appropriate fertilization.
1.1.3. Fruit
The only fruit that has been studied is the apple, which contained 0.0023 μg of KYNA/g wet weight [73].
1.1.4. Spices and Herbs for Cooking
KYNA content in spices and herbs for cooking was determined in 19 different products. The highest amount of KYNA was found in basil and thyme, 14.08 and 8.87 μg/g wet weight, respectively [77]. The numbers seem to be relatively high in comparison to plants. However, it must be stressed that, generally, commercially marketable spices are dried. Therefore, it is hard to accurately compare the concentration of KYNA in spices and fresh plants.
1.1.5. Honey
Unexpectedly, honey and other bee products contain relatively large amounts of KYNA (Figure 3). An extremely high amount of KYNA was found in chestnut honey, up to 2114.9 μg/g [74]. Interestingly, this applies to chestnut honey obtained from different locations in Europe and Korea [13,74,80]. This is a phenomenon even among other types of honey. KYNA content in popular honey such as sunflower, multiflorous, buckwheat, acacia, and linden honey is 1.73, 0.877, 0.33, 0.181, and 0.179 μg/g, respectively. A high level of KYNA in chestnut honey seems to be related to the high KYNA content in male flowers of the chestnut tree [13].
1.1.6. Dairy
In commercially available dairy products, the content of KYNA is as follows: cow’s milk: 0.017 μg/mL, kefir: 0.2417 μg/mL, yoghurt: 0.2868 μg/mL, white cheese: 0.0766 μg/g, and hard cheese: 0.0084 [42,49] (Figure 3 [73,78].
1.1.7. Fermented Food and Beverages
A relatively high KYNA content was found in fermented food products (Figure 3). It was found in kefir and yoghurt in amounts of up to 0.242 and 0.287 μg/mL, respectively [73,78]. In addition, cocoa powder contains KYNA in the amount of 4.486 μg/g [78]. Interestingly, the alcoholic beverages wine and beer contain KYNA in a broad range of concentrations, up to 0.179 and 0.051 μg/mL, respectively [11,78,79]. These results indicate that the fermenting microorganisms produce KYNA and that this process may significantly increase the KYNA content in food and beverages.
1.1.8. Medicinal Herbs and Supplements
The presence of KYNA has been demonstrated in medicinal herbs and supplements. The highest content expressed on a dry weight basis of the herbs was found in leaves of peppermint, nettle, birch, and horsetail, ranging from 2.27 to 3.82 μg/g dry weight [83]. The intake of KYNA in herbal infusions prepared according to manufacturer’s instructions were found to vary from 1.08 μg/day in the nettle root infusion to 32.5 and 32.6 μg/day in the nettle leaf and St. John’s wort infusion, respectively. Herbal supplements in the form of tablets also contain KYNA. KYNA delivery calculated in a maximum recommended dose of the supplement equals from 0.41 to 30.38 μg/g in chamomile and St. John’s wort tablets, respectively [82].
1.1.9. Baby Food
KYNA was found in human milk [45,46,73]. Interestingly, the content of KYNA in human milk increases more than 14 times during the time of breastfeeding, starting from 0.004 μg/mL on day 3 after labor and reaching a value of 0.057 μg/mL in the 6th month of feeding [46]. KYNA was also found in all 46 artificial baby milk formulas studied. However, in comparison with human milk, in which its content naturally changed over time, the concentration of KYNA in artificial formulas was substantially lower and did not follow its physiological dynamics of changes [46]. In first-food formulas, KYNA content is clearly higher in products containing vegetables (0.0056–0.0148 µg/g) than in meat-based food (0.01 µg/g). In fruit and vegetable juice, its concentration is 0.0019 µg/g [73]. It should be noted that this estimation is based on single measurement results only, which are insufficient to draw definite conclusions.
1.2. Animal Food
The presence of KYNA was studied in animal feed for livestock, cats, dogs, and fish. It was shown that KYNA is present in animal feed in varying concentrations. The highest concentration of KYNA was found in feed for livestock, where it varied from 0.198 μg/g fresh weight to 0.414 μg/g fresh weight. Based on the measurement of KYNA content in feed ingredients, the authors concluded that the concentration of KYNA in animal feed was not controlled and deliberately set, but its final content depended on the ingredients used [84].
2. Kynurenic Acid Supplementation
2.1. Health Effects of Kynurenic Acid Supplementation
Since KYNA is formed endogenously in the body and can also be supplied in food, the question about the relevance of its supplementation is legitimate. No human studies devoted to health effects of exogenously administered KYNA have been conducted to date. An exception is a study in which a solution of chestnut honey was administered to young volunteers and pharmacokinetic parameters of KYNA were determined afterwards. No side effects were reported in this study [11]. Indirectly, the lack of KYNA toxicity can be inferred from studies in which tryptophan was administered to humans. It was found that tryptophan load resulted in an increase in kynurenines, including KYNA, in the blood. Very recently, Sathyasaikumar et al. (2022) reported no serious adverse events and no long-term changes in behavior and health in tryptophan-treated humans who had plasma KYNA levels that increased as much as 145-fold compared to pre-tryptophan values [85].
Further conclusions should be drawn based on the data obtained from studies on rodents. The data search performed showed that there are few publications describing KYNA administration via the alimentary route for the period lasting from 3 days to 2 months (Table 2).
Table 2. Consequences of kynurenic acid (KYNA) dietary supplementation in rodents.
| Species | KYNA Treatment (Dose, Schedule) |
Effect/Properties | Reference |
|---|---|---|---|
| Adult animals | |||
| Rats, mice | 25 or 250 mg/L in drinking water for 3–21 days |
|
[86] |
| Mice | 2.5, 25, or 250 mg/L in drinking water for 3, 7, 14, 28 days |
|
[87] |
| Mice | 2.5, 25, or 250 mg/L in drinking water for 7–14 days |
|
[88] |
| Spontaneously hypertensive rats | 25 mg/kg/day in drinking water for 3 weeks |
|
[89] |
| Mice | 5 mg/kg/day, intragastric; once a day for 8 weeks |
High-fat diet induced:
|
[90] |
| Young animals | |||
| Rats | 25 or 250 mg/L in drinking water; from PND 1 to PND 60 |
|
[91] |
| Rats | 250 mg/L in drinking water; from PND 1 to PND 21 |
|
[46] |
| Rats | 25 mg/L in drinking water; from PND 21 until 9th week of life |
|
[92] |
Because KYNA is water-soluble, it was administered in drinking water in most studies. This mode of administration is very convenient because it does not cause stress to the animal. In addition, water was available ad libitum, which allowed KYNA to be taken in the most natural way according to the daily pattern of drinking. The concentrations used ranged from 2.5 to 250 mg/L, approximating a dose of 0.25 to 25 mg/kg of KYNA per 1 kg body weight/day, respectively. Studies have been conducted on both young and adult animals. Generally, no toxic effects of KYNA administration have been reported. When administered to young animals, KYNA did not interfere with their overall growth and development. However, a moderate reduction in the rate of weight gain was observed. This effect was evident in young, but not adult, animals. On the other hand, the reduction of weight gain rate was present in adult rats kept on a high-fat diet (Table 2). These observations allowed for hypothesizing antiobesogenic properties of KYNA during early development [46]. Interestingly, KYNA has been found to stimulate intestinal mucosal growth in young rats and to cause, inter alia, an increase in the intestinal surface area. However, this affects neither the body composition nor bone mineralization and endurance capacity (Table 2). Importantly, the supplementation of KYNA in drinking water to developing rats for 2 months did not impair their brain functions measured in adulthood [92].
In healthy adult mice, the alimentary administration of KYNA did not affect blood hematological parameters. However, experiments performed in vitro on leukocytes and splenocytes obtained from drug supplemented animals revealed that KYNA exerted antioxidant and immunomodulatory effects [87,88]. In spontaneously hypertensive adult rats, the administration of KYNA in drinking water for 3 weeks did not affect the mean arterial pressure, but it moderately reduced heart rate [89] (Table 2).
Another method of the alimentary administration of the drug was utilized by Li et al. (2021) [90]. In this study, KYNA was applied intragastrically to adult mice kept on a high-fat diet, once a day for 8 weeks, in a dose of 5 mg/kg/day. It was found that such a regimen resulted in declined body weight gain and reduced daily energy intake. Moreover, the following serum metabolic parameters were improved: triglyceride, serum high-density lipoprotein cholesterol, and low-density lipoprotein levels. Finally, both the atherosclerosis index and the coronary artery risk index were significantly decreased [90]. Similar metabolic effects of KYNA injected intraperitoneally at a dose of 5 mg/kg/day, once a day for 1 to 4 weeks, to mice on a high-fat diet were described [93]. Very recently, it was reported that KYNA administered intraperitoneally three times decreased the colonization of the intestine by fungi and ameliorated intestinal injury, i.e., inhibited inflammation, promoted the expression of intestinal tight junction proteins, and protected from intestinal barrier damage caused by invasive Candida albicans infection in mice [94] (Table 2). Other data indicating multiple health-promoting effects of KYNA come from studies in which the drug was acutely injected intraperitoneally (Table S7). This route of administration is preferred by researchers because it allows for the accurate dosage and precise determination of the time of action of the substance. Historically, the earliest data relate to the antiulcer effects of KYNA. Glavin and Pinsky, in 1989, showed that KYNA significantly blocked restraint-cold stress ulcers, ethanol ulcers, and basal nonstimulated gastric acid secretion in normal rats. The authors premised both peripheral and cerebral effects of KYNA [95]. Protective effects of injected KYNA on the liver and the pancreas, and in disorders of the lower gastrointestinal tract, were later described (Table S7). More recently, numerous reports performed on animals on the beneficial effect of KYNA on the conditions commonly referred to as metabolic diseases in humans were published. Antiobesity, cholesterol-lowering, glucose tolerance improvement, and antiatherosclerotic effects were evidenced in appropriate animal models (Table S7). It is surprising that many of these effects observed after KYNA administration in animals can be therapeutic targets for metabolic syndromes in humans (Figure 4).
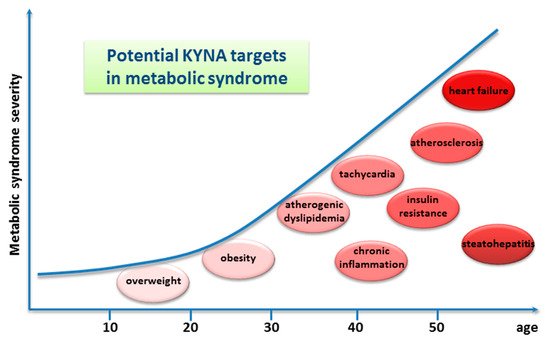
Figure 4. Graphic presentation of potential kynurenic acid (KYNA) targets in metabolic syndrome in humans. The graphic is based on [96]; however, only identified KYNA targets are presented.
Similar disturbances were described as obese-related multimorbidity, when a low-caloric KYNA-enriched functional diet should be considered as supportive care. The effect of KYNA on bone metabolism is also of interest. A recent publication by Shi et al. (2022) revealed that KYNA administered in a relatively low dose of 5 mg/kg/day for 4 weeks alleviated the postmenopausal osteoporosis and highlighted the involvement of the GPR35 receptor, a molecular target of KYNA, in this action [97].
In addition, it has been shown that KYNA may have beneficial health effects in life-threatening conditions, and this issue requires a separate commentary. Moroni et al. (2012) were the first to communicate that KYNA administered subcutaneously at doses of 500 mg/kg (single injection) or 200 mg/kg three times at 0, 3, and 6 h after LPS dramatically reduced LPS-induced death in mice [98]. The Hungarian group confirmed that KYNA protects against LPS-induced sepsis in subsequent publications, in which a much lower KYNA dose of 30 mg/kg, i.p., was used [99,100]. Most recently, Wang et al. (2022) demonstrated that intraperitoneally administered KYNA (5 mg/kg; three times at days 3, 6, and 9) reduced the mortality of mice infected with Candida albicans [94]. Hsieh et al. (2011) reported that KYNA administered intravenously at doses ranging from 30–100 mg/kg attenuated multiorgan dysfunction in rats exposed to heatstroke [101]. Kaszaki et al. (2008) found a profound anti-inflammatory action of KYNA administered in intravenous infusion in experimental colon obstruction in dogs [102]. Similarly, Marciniak, in 2013, demonstrated that the intravenous infusion of KYNA alleviated symptoms of experimental acute pancreatitis in rats [103]. These results deserve special attention because they indicate the feasibility of using KYNA administered as a bolus or an intravenous infusion in life-threatening conditions.
The beneficial wound healing effects of KYNA after its external administration on the skin and cornea in rabbits are also worth mentioning [104,105]. It is worth noting that a clinical trial with the use of 0.5% KYNA dressing in people with skin scarring has already been successfully completed [106]. Furthermore, KYNA encapsulated in synthetic polymer microspheres implanted in a wound bed in rats was shown to reduce fibrotic tissue formation [107].
Since there are no substantial differences between the effects exerted by KYNA administered alimentary or infused by injection, it can be assumed that its supplementation by the oral route will produce similar effects as those described after infusion.
2.2. Clinical Trials
In the ClinicalTrials.gov database, only four registered studies were found in which KYNA was applied to humans. All studies were dedicated to examining the effect of the topical application of KYNA to skin.
2.3. Patents
A survey of the PubChem database of patents in which the keyword KYNA appears revealed 23 patent applications dealing with the medical use of the drug. Most of them relate to its oral or injectable administration and only a few assume the topical or local administration of KYNA. The most commonly claimed effect of KYNA is in digestive tract, liver, and pancreatic conditions. KYNA’s effects have also been recommended in cardiovascular pathologies, lipid metabolism disorders, obesity, and kidney dysfunction. In addition, its use in fibrotic diseases, eye diseases, and mental stress is mentioned. In general, this is in line with the data obtained from scientific publications. Some novelty derived from the patent descriptions is KYNA’s effect on skeletal muscles and its proposed use in sarcopenia or hangover control. In addition to the scientific content, it is worth noting that the number of patent applications on the medical use of KYNA has increased substantially since 2016.
This entry is adapted from the peer-reviewed paper 10.3390/nu14194182
This entry is offline, you can click here to edit this entry!
