Ischemic stroke is a leading cause of death worldwide, mainly in western countries. So far, approved therapies rely mainly on reperfusion of the affected brain area, by intravenous thrombolysis or mechanical thrombectomy. The combination of pharmacological brain-protective strategies with reperfusion is the future of stroke therapy, aiming to reduce brain cell death and decrease patients’ disabilities. The success of new therapies relies on bringing preclinical studies and clinical practice close together. Recent upgrades of in vitro and in vivo stroke models for accurate and effective evaluation of therapeutic strategies are described.
- ischemic stroke
- cytoprotection
- spheroids
- organoids
- MCAO
- preclinical
- therapies
1. Stroke
2. Ischemic Stroke
2.1. Risk Factors
2.2. Diagnosis
2.3. Key Concept: Core and Penumbra
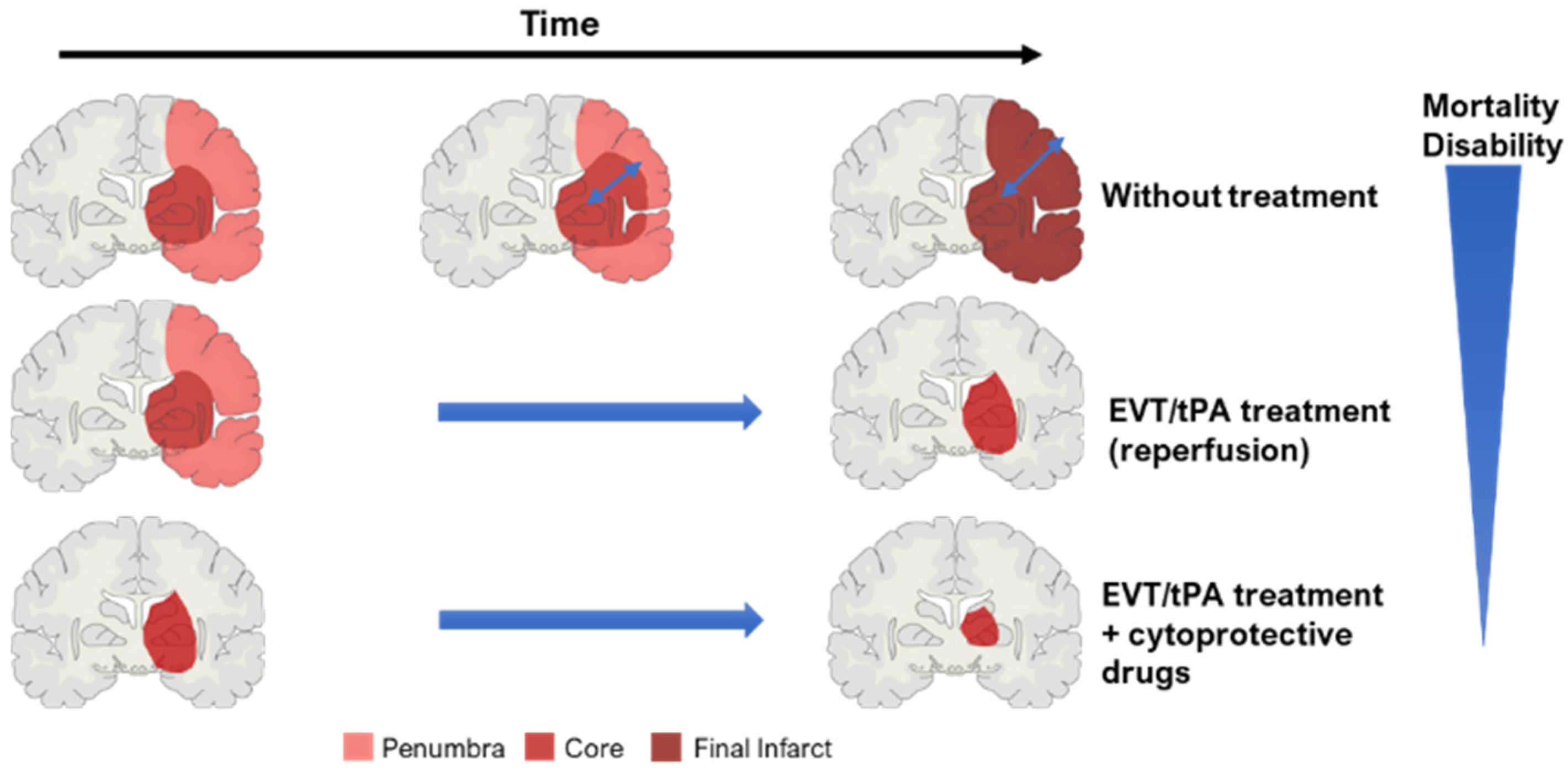
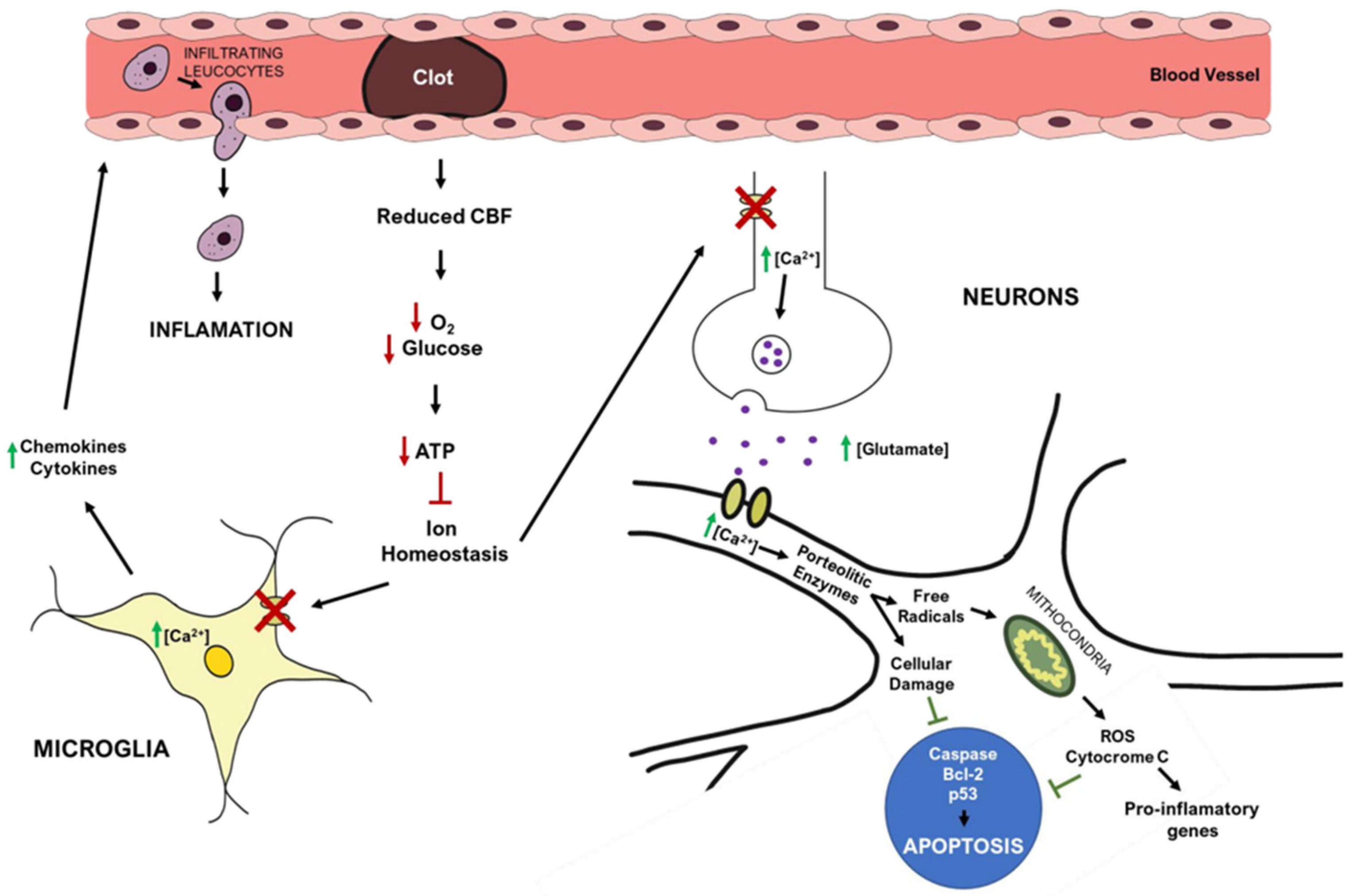
2.4. Ischemic Stroke Pathophysiology
2.5. Treatments
2.5.1. Thrombolysis
2.5.2. Mechanical Thrombectomy
2.5.3. Neuroprotective Drugs
| Clinical Trial | Therapeutic Drug | Mechanism of Action | Status of Clinical Trial |
|---|---|---|---|
| VENUS (Phase III) | Nimodipine | Blocks voltage-gated channels (calcium) | Terminated: not effective |
| MAVARIC (Phase I) | Verapamil (with magnesium sulfate) | Blocks of voltage-gated channels (calcium), after reperfusion | Results not published yet |
| ESCAPE-NA1 (Phase III) | Nerinetide (Na-1) | Inhibits neuronal excitotoxicity | Approved by American FDA alone; but it does not display protection together with IVT |
| ESCAPE-NEXT (Phase III) | Nerinetide (Na-1) together with EVT therapy | Inhibits neuronal excitotoxicity | Ongoing (completes in August 2023) |
| URICO-ICTUS (Phase III) | Uric Acid (UA) | Antioxidant agent (reduces inflammation) | Small study sample, but a confirmatory trial is planned |
| BAST (Phase III) | 3-N-butylphtalide (NBP) | Free radical scavenger (reduces inflammation) | Ongoing (completed on 31 December 2022), good outcome so far |
| Evaluation of HUK in AIS (Phase IV) | Human Urinary Kallidinogenase (HUK) | Anti-inflammatory agents suppress TLR4/NF-kB pathway | Approved by China’s FDA, with post-approval surveillance ongoing |
| A study of allogeneic mesenchymal bone marrow cells in AIS (Phase I/II) | Allogeneic adult mesenchymal bone marrow cells | Cell-based therapy | Completed, presented beneficial behavioral outcomes for patients |
| J-REPAIR (Phase I/II) | JTR-161 (allogeneic stem cell product) | Cell-based therapy | Last updated on June 2022, results not published yet |
| Efficacy and safety of FTY720 for AIS (phase II) | Fingolimod (FTY720) | Immune modulator | Completed; reduced injury and improved clinical outcomes |
| Combination of the immune modulator Dimethyl Fumarate with alteplase in AIS (phase I/II) | Dimethyl Fumarate | Immune modulator | Ongoing (completes in December 2022) |
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102561
References
- Rtf, C. A Systematic Approach to the Definition of Stroke. Austin J. Cerebrovasc. Dis. Stroke 2014, 1, 1024.
- Coupland, A.P.; Thapar, A.; Qureshi, M.I.; Jenkins, H.; Davies, A.H. The Definition of Stroke. J. R. Soc. Med. 2017, 110, 9–12.
- Sacco, R.L.; Kasner, S.E.; Broderick, J.P.; Caplan, L.R.; Connors, J.J.; Culebras, A.; Elkind, M.S.V.; George, M.G.; Hamdan, A.D.; Higashida, R.T.; et al. An Updated Definition of Stroke for the 21st Century: A Statement for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke 2013, 44, 2064–2089.
- Campbell, B.C.V.; Khatri, P. Stroke. Lancet 2020, 396, 129–142.
- Lipton, P. Ischemic Cell Death in Brain Neurons. Physiol. Rev. 1999, 79, 1431–1568.
- Murphy, S.J.; Werring, D.J. Stroke: Causes and Clinical Features. Medicine 2020, 48, 561–566.
- Katan, M.; Luft, A. Global Burden of Stroke. Semin. Neurol. 2018, 38, 208–211.
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609.
- Tuo, Q.-z.; Zhang, S.-t.; Lei, P. Mechanisms of Neuronal Cell Death in Ischemic Stroke and Their Therapeutic Implications. In Medicinal Research Reviews; John Wiley and Sons Inc.: Hoboken, NJ, USA, 2021.
- Johnson, C.O.; Nguyen, M.; Roth, G.A.; Nichols, E.; Alam, T.; Abate, D.; Abd-Allah, F.; Abdelalim, A.; Abraha, H.N.; Abu-Rmeileh, N.M.; et al. Global, Regional, and National Burden of Stroke, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 439–458.
- Wafa, H.A.; Wolfe, C.D.A.; Emmett, E.; Roth, G.A.; Johnson, C.O.; Wang, Y. Burden of Stroke in Europe: Thirty-Year Projections of Incidence, Prevalence, Deaths, and Disability-Adjusted Life Years. Stroke 2020, 51, 2418–2427.
- Stevens, E.; Emmett, E.; Wang, Y.; McKevitt, C.; Wolfe, C.D.A. The Burden of Stroke in Europe: Report; Stroke Alliance for Europe: City Road, London, 2017.
- Kisling, L.A.; Das, J.M. Prevention Strategies; StatPearls Publ: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK537222/ (accessed on 4 May 2022).
- Ntaios, G. Embolic Stroke of Undetermined Source: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2020, 75, 333–340.
- Pierik, R.; Algra, A.; van Dijk, E.; Erasmus, M.E.; van Gelder, I.C.; Koudstaal, P.J.; Luijckx, G.R.; Nederkoorn, P.J.; van Oostenbrugge, R.J.; Ruigrok, Y.M.; et al. Distribution of Cardioembolic Stroke: A Cohort Study. Cere 2020, 49, 97–104.
- Sommer, C.J. Ischemic Stroke: Experimental Models and Reality. Acta Neuropathol. 2017, 133, 245–261.
- Gormley, G.J. Chemoprevention Strategies. J. Cell. Biochem. 1992, 50, 106.
- Grysiewicz, R.A.; Thomas, K.; Pandey, D.K. Epidemiology of Ischemic and Hemorrhagic Stroke: Incidence, Prevalence, Mortality, and Risk Factors. Neurol. Clin. 2008, 26, 871–895.
- National Institute of Health. Stroke|NHLBI, NIH; National Heart, Lung and Blood institute: Bethesda, MD, USA, 2008.
- Avan, A.; Digaleh, H.; Di Napoli, M.; Stranges, S.; Behrouz, R.; Shojaeianbabaei, G.; Amiri, A.; Tabrizi, R.; Mokhber, N.; Spence, J.D.; et al. Socioeconomic Status and Stroke Incidence, Prevalence, Mortality, and Worldwide Burden: An Ecological Analysis from the Global Burden of Disease Study 2017. BMC Med. 2019, 17, 191.
- Howard, V.J.; Madsen, T.E.; Kleindorfer, D.O.; Judd, S.E.; Rhodes, J.D.; Soliman, E.Z.; Kissela, B.M.; Safford, M.M.; Moy, C.S.; McClure, L.A.; et al. Sex and Race Differences in the Association of Incident Ischemic Stroke with Risk Factors. JAMA Neurol. 2019, 76, 179–186.
- Floßmann, E.; Schulz, U.G.R.; Rothwell, P.M. Systematic Review of Methods and Results of Studies of the Genetic Epidemiology of Ischemic Stroke. Stroke 2004, 35, 212–227.
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495.
- Deb, P.; Sharma, S.; Hassan, K.M. Pathophysiologic Mechanisms of Acute Ischemic Stroke: An Overview with Emphasis on Therapeutic Significance beyond Thrombolysis. Pathophysiology 2010, 17, 197–218.
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654.
- Barthels, D.; Das, H. Current Advances in Ischemic Stroke Research and Therapies. Biochim. Biophys. Acta—Mol. Basis Dis. 2020, 1866, 165260.
- Rosamond, W.; Flegal, K.; Furie, K.; Go, A.; Greenlund, K.; Haase, N.; Hailpern, S.M.; Ho, M.; Howard, V.; Kissela, B.; et al. Heart Disease and Stroke Statistics-2008 Update: A Report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation 2008, 117, e25–e146.
- Virani, S.S.; Alonso, A.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics—2020 Update: A Report from the American Heart Association. In Circulation; Lippincott Williams & Wilkins: Hagerstown, MD, USA, 2020; pp. E139–E596.
- WHO. WHO|Stroke, Cerebrovascular Accident|Health Topics; World Health Organization: Geneva, Switzerland, 2022.
- Feske, S.K. Ischemic Stroke. Am. J. Med. 2021, 134, 1457–1464.
- Randolph, S.A. Ischemic Stroke. Work. Health Saf. 2016, 64, 444.
- Ojaghihaghighi, S.; Vahdati, S.S.; Mikaeilpour, A.; Ramouz, A. Comparison of Neurological Clinical Manifestation in Patients with Hemorrhagic and Ischemic Stroke. World J. Emerg. Med. 2017, 8, 34.
- Kuhrij, L.S.; Marang-Van De Mheen, P.J.; Van Den Berg-Vos, R.M.; De Leeuw, F.E.; Nederkoorn, P.J.; Lingsma, H.F.; De Borst, G.J.; Van Norden, A.G.W.; Eysink Smeets, M.M.; Aerden, L.A.M.; et al. Determinants of Extended Door-to-Needle Time in Acute Ischemic Stroke and Its Influence on in-Hospital Mortality: Results of a Nationwide Dutch Clinical Audit. BMC Neurol. 2019, 19, 265.
- Jamieson, D.G. Diagnosis of Ischemic Stroke. Am. J. Med. 2009, 122 (Suppl. S2), S14–S20.
- Phipps, M.S.; Cronin, C.A. Management of Acute Ischemic Stroke. BMJ 2020, 368, l6983.
- Puig, J.; Shankar, J.; Liebeskind, D.; Terceño, M.; Nael, K.; Demchuk, A.M.; Menon, B.; Dowlatshahi, D.; Leiva-Salinas, C.; Wintermark, M.; et al. From “Time Is Brain” to “Imaging Is Brain”: A Paradigm Shift in the Management of Acute Ischemic Stroke. J. Neuroimaging 2020, 30, 562–571.
- Calderon, V.J.; Kasturiarachi, B.M.; Lin, E.; Bansal, V.; Zaidat, O.O. Review of the Mobile Stroke Unit Experience Worldwide. Interv. Neurol. 2018, 7, 347–358.
- Helwig, S.A.; Ragoschke-Schumm, A.; Schwindling, L.; Kettner, M.; Roumia, S.; Kulikovski, J.; Keller, I.; Manitz, M.; Martens, D.; Grün, D.; et al. Prehospital Stroke Management Optimized by Use of Clinical Scoring vs Mobile Stroke Unit for Triage of Patients with Stroke: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 1484–1492.
- Kiewert, C.; Mdzinarishvili, A.; Hartmann, J.; Bickel, U.; Klein, J. Metabolic and Transmitter Changes in Core and Penumbra after Middle Cerebral Artery Occlusion in Mice. Brain Res. 2010, 1312, 101–107.
- Horváth, E.; Huțanu, A.; Chiriac, L.; Dobreanu, M.; Orădan, A.; Nagy, E.E. Ischemic Damage and Early Inflammatory Infiltration Are Different in the Core and Penumbra Lesions of Rat Brain after Transient Focal Cerebral Ischemia. J. Neuroimmunol. 2018, 324, 35–42.
- Shi, L.; Rocha, M.; Leak, R.K.; Zhao, J.; Bhatia, T.N.; Mu, H.; Wei, Z.; Yu, F.; Weiner, S.L.; Ma, F.; et al. A New Era for Stroke Therapy: Integrating Neurovascular Protection with Optimal Reperfusion. J. Cereb. Blood Flow Metab. 2018, 38, 2073–2091.
- Uzdensky, A.B. Apoptosis Regulation in the Penumbra after Ischemic Stroke: Expression of pro- and Antiapoptotic Proteins. In Apoptosis; Springer: Berlin/Heidelberg, Germany, 2019; pp. 687–702.
- Pawluk, H.; Woźniak, A.; Grześk, G.; Kołodziejska, R.; Kozakiewicz, M.; Kopkowska, E.; Grzechowiak, E.; Kozera, G. The Role of Selected Pro-Inflammatory Cytokines in Pathogenesis of Ischemic Stroke. In Clinical Interventions in Aging; Dove Press: Macclesfield, UK, 2020; pp. 469–484.
- Guo, Y.; Li, P.; Guo, Q.; Shang, K.; Yan, D.; Du, S.; Lu, Y. Pathophysiology and Biomarkers in Acute Ischemic Stroke—A Review. Trop. J. Pharm. Res. 2013, 12, 1097–1105.
- Mergenthaler, P.; Dirnagl, U.; Kunz, A. Ischemic Stroke: Basic Pathophysiology and Clinical Implication. In Neuroscience in the 21st Century—From Basic to Clinical; Springer: New York, NY, USA, 2013; pp. 2543–2563, Chapter 97.
- Singhal, A.B.; Lo, E.H.; Dalkara, T.; Moskowitz, M.A. Ischemic Stroke: Basic Pathophysiology and Neuroprotective Strategies. Acute Ischemic Stroke—Imaging and Intervention; Springer: Berlin/Heidelberg, Germany, 2011; pp. 1–24, Chapter 1.
- Nour, M.; Scalzo, F.; Liebeskind, D.S. Ischemia-Reperfusion Injury in Stroke. Interv. Neurol. 2013, 1, 185–199.
- Campbell, B.C.V.; Meretoja, A.; Donnan, G.A.; Davis, S.M. Twenty-Year History of the Evolution of Stroke Thrombolysis with Intravenous Alteplase to Reduce Long-Term Disability. Stroke 2015, 46, 2341–2346.
- Molina, C.A.; Saver, J.L. Extending Reperfusion Therapy for Acute Ischemic Stroke: Emerging Pharmacological, Mechanical, and Imaging Strategies. Stroke 2005, 36, 2311–2320.
- Bhaskar, S.; Stanwell, P.; Cordato, D.; Attia, J.; Levi, C. Reperfusion Therapy in Acute Ischemic Stroke: Dawn of a New Era? BMC Neurol. 2018, 18, 1–26.
- Daniel, M.; Goel, I.; Sakai, Y.; Teramura, Y. Current Status of Ischemic Stroke Treatment: From Thrombolysis to Potential Regenerative Medicine. Regen. Ther. 2021, 18, 408–417.
- Herpich, F.; Rincon, F. Management of Acute Ischemic Stroke. Crit. Care Med. 2020, 48, 1654–1663.
- Campbell, B.C.V.; Mitchell, P.J.; Churilov, L.; Yassi, N.; Kleinig, T.J.; Dowling, R.J.; Yan, B.; Bush, S.J.; Dewey, H.M.; Thijs, V.; et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. N. Engl. J. Med. 2018, 378, 1573–1582.
- Berge, E.; Whiteley, W.; Audebert, H.; De Marchis, G.M.; Fonseca, A.C.; Padiglioni, C.; de la Ossa, N.P.; Strbian, D.; Tsivgoulis, G.; Turc, G. European Stroke Organisation (ESO) Guidelines on Intravenous Thrombolysis for Acute Ischaemic Stroke. Eur. Stroke J. 2021, 6, I.
- Seners, P.; Caroff, J.; Chausson, N.; Turc, G.; Denier, C.; Piotin, M.; Aghasaryan, M.; Alecu, C.; Chassin, O.; Lapergue, B.; et al. Recanalization before Thrombectomy in Tenecteplase vs. Alteplase-Treated Drip-and-Ship Patients. J. Stroke 2019, 21, 105–107.
- Papanagiotou, P.; Ntaios, G. Endovascular Thrombectomy in Acute Ischemic Stroke. Circ. Cardiovasc. Interv. 2018, 11, e005362.
- Blanc, R.; Escalard, S.; Baharvadhat, H.; Desilles, J.P.; Boisseau, W.; Fahed, R.; Redjem, H.; Ciccio, G.; Smajda, S.; Maier, B.; et al. Recent Advances in Devices for Mechanical Thrombectomy. Expert Rev. Med. Devices 2020, 17, 697–706.
- Maurice, A.; Ferré, J.C.; Ronzière, T.; Devys, J.M.; Subileau, A.; Laffon, M.; Laviolle, B.; Beloeil, H. GASS Trial Study Protocol: A Multicentre, Single-Blind, Randomised Clinical Trial Comparing General Anaesthesia and Sedation during Intra-Arterial Treatment for Stroke. BMJ Open 2019, 9, e024249.
- Tsivgoulis, G.; Katsanos, A.H. Important Advances in Stroke Research in 2020. Lancet Neurol. 2021, 20, 2–3.
- Yang, P.; Zhang, Y.; Zhang, L.; Zhang, Y.; Treurniet, K.M.; Chen, W.; Peng, Y.; Han, H.; Wang, J.; Wang, S.; et al. Endovascular Thrombectomy with or without Intravenous Alteplase in Acute Stroke. N. Engl. J. Med. 2020, 382, 1981–1993.
- Katsanos, A.H.; Malhotra, K.; Goyal, N.; Arthur, A.; Schellinger, P.D.; Köhrmann, M.; Krogias, C.; Turc, G.; Magoufis, G.; Leys, D.; et al. Intravenous Thrombolysis Prior to Mechanical Thrombectomy in Large Vessel Occlusions. Ann. Neurol. 2019, 86, 395–406.
- Nogueira, R.G.; Tsivgoulis, G. Large Vessel Occlusion Strokes after the DIRECT-MT and SKIP Trials: Is the Alteplase Syringe Half Empty or Half Full? Stroke 2020, 51, 3182–3186.
- Jeyaseelan, K.; Lim, K.Y.; Armugam, A. Neuroprotectants in Stroke. Pharmacotherapy 2008, 9, 887–900.
- DeGraba, T.J.; Pettigrew, L.C. Why Do Neuroprotective Drugs Work in Animals but Not Humans? Neurol. Clin. 2000, 18, 475–493.
- Dirnagl, U.; Priller, J. Focal Cerebral Ischemia: The Multifaceted Role of Glial Cells. In Neuroglia; Oxford Academic: Oxford, UK, 2013.
- Nedergaard, M.; Dirnagl, U. Role of Glial Cells in Cerebral Ischemia. Glia 2005, 50, 281–286.
- Wang, L.; Xiong, X.; Zhang, L.; Shen, J. Neurovascular Unit: A Critical Role in Ischemic Stroke. CNS Neurosci. Ther. 2021, 27, 7–16.
- Luo, Y.; Tang, H.; Li, H.; Zhao, R.; Huang, Q.; Liu, J. Recent Advances in the Development of Neuroprotective Agents and Therapeutic Targets in the Treatment of Cerebral Ischemia. Eur. J. Med. Chem. 2019, 162, 132–146.
- Lapchak, P.A.; Zhang, J.H. The High Cost of Stroke and Stroke Cytoprotection Research. Transl. Stroke Res. 2016, 8, 307–317.
- Fisher, M.; Savitz, S.I. Pharmacological Brain Cytoprotection in Acute Ischaemic Stroke—Renewed Hope in the Reperfusion Era. Nat. Rev. Neurol. 2022, 18, 193–202.
- Cheng, Y.D.; Al-Khoury, L.; Zivin, J.A. Neuroprotection for Ischemic Stroke: Two Decades of Success and Failure. NeuroRx 2004, 1, 36.
- Das, J.M.; Zito, P.M. Nimodipine. In xPharm: The Comprehensive Pharmacology Reference; Elsevier Inc.: Amsterdam, The Netherlands, 2021; pp. 1–7.
- Horn, J.; De Haan, R.J.; Vermeulen, M.; Limburg, M. Very Early Nimodipine Use in Stroke (VENUS). Stroke 2001, 32, 461–465.
- Magnesium and Verapamil after Recanalization in Ischemia of the Cerebrum: A Clinical and Translational Study (MAVARIC; NCT02912663). Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT02912663&cntry=&state=&city=&dist= (accessed on 7 June 2022).
- Saver, J.L.; Starkman, S.; Eckstein, M.; Stratton, S.J.; Pratt, F.D.; Hamilton, S.; Conwit, R.; Liebeskind, D.S.; Sung, G.; Kramer, I.; et al. Prehospital Use of Magnesium Sulfate as Neuroprotection in Acute Stroke. N. Engl. J. Med. 2015, 372, 528–536.
- Cigel, A.; Sayin, O.; Gurgen, S.G.; Sonmez, A. Long Term Neuroprotective Effects of Acute Single Dose MK-801treatment against Traumatic Brain Injury in Immature Rats. Neuropeptides 2021, 88, 102161.
- Sattler, R.; Xiong, Z.; Lu, W.Y.; Hafner, M.; MacDonald, J.F.; Tymianski, M. Specific Coupling of NMDA Receptor Activation to Nitric Oxide Neurotoxicity by PSD-95 Protein. Science 1999, 284, 1845–1848.
- Ugalde-Triviño, L.; Díaz-Guerra, M. PSD-95: An Effective Target for Stroke Therapy Using Neuroprotective Peptides. Int. J. Mol. Sci. 2021, 22, 12585.
- Aarts, M.; Liu, Y.; Liu, L.; Besshoh, S.; Arundine, M.; Gurd, J.W.; Wang, Y.T.; Salter, M.W.; Tymianski, M. Treatment of Ischemic Brain Damage by Perturbing NMDA Receptor-PSD-95 Protein Interactions. Science 2002, 298, 846–850.
- Hill, M.D.; Goyal, M.; Menon, B.K.; Nogueira, R.G.; McTaggart, R.A.; Demchuk, A.M.; Poppe, A.Y.; Buck, B.H.; Field, T.S.; Dowlatshahi, D.; et al. Efficacy and Safety of Nerinetide for the Treatment of Acute Ischaemic Stroke (ESCAPE-NA1): A Multicentre, Double-Blind, Randomised Controlled Trial. Lancet 2020, 395, 878–887.
- Fisher, M.; Israel, B.; Xiong, Y.; Wakhloo, A.K. Advances in Acute Ischemic Stroke Therapy. Circ. Res. 2022, 130, 1230–1251.
- Efficacy and Safety of Nerinetide in Participants with Acute Ischemic Stroke Undergoing Endovascular Thrombectomy Excluding Thrombolysis.(ESCAPE-NEXT; NCT04462536). Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT04462536&cntry=&state=&city=&dist= (accessed on 7 June 2022).
- Bennion, D.M.; Isenberg, J.D.; Harmel, A.T.; DeMars, K.; Dang, A.N.; Jones, C.H.; Pignataro, M.E.; Graham, J.T.; Steckelings, U.M.; Alexander, J.C.; et al. Post-Stroke Angiotensin II Type 2 Receptor Activation Provides Long-Term Neuroprotection in Aged Rats. PLoS ONE 2017, 12, e0180738.
- Eklund, P.C.; Långvik, O.K.; Wärnå, J.P.; Salmi, T.O.; Willför, S.M.; Sjöholm, R.E. Chemical Studies on Antioxidant Mechanisms and Free Radical Scavenging Properties of Lignans. Org. Biomol. Chem. 2005, 3, 3336–3347.
- Aliena-Valero, A.; Rius-Pérez, S.; Baixauli-Martín, J.; Torregrosa, G.; Chamorro, Á.; Pérez, S.; Salom, J.B. Uric Acid Neuroprotection Associated to IL-6/STAT3 Signaling Pathway Activation in Rat Ischemic Stroke. Mol. Neurobiol. 2020, 58, 408–423.
- Chamorro, Á.; Amaro, S.; Castellanos, M.; Segura, T.; Arenillas, J.; Martí-Fábregas, J.; Gállego, J.; Krupinski, J.; Gomis, M.; Cánovas, D.; et al. Safety and Efficacy of Uric Acid in Patients with Acute Stroke (URICO-ICTUS): A Randomised, Double-Blind Phase 2b/3 Trial. Lancet Neurol. 2014, 13, 453–460.
- Chamorro, Á.; Lo, E.H.; Renú, A.; Van Leyden, K.; Lyden, P.D. The Future of Neuroprotection in Stroke. J. Neurol. Neurosurg. Psychiatry 2021, 92, 129–135.
- Efficacy and Safety of Butylphthalide for Acute Ischemic Stroke Patients Receiving Intravenous Thrombolysis or Endovascular Treatment. (BAST; NCT03539445). Available online: https://clinicaltrials.gov/ct2/results?cond=&term=NCT03539445&cntry=&state=&city=&dist= (accessed on 7 June 2022).
- Yamaguchi, T.; Sano, K.; Takakura, K.; Saito, I.; Shinohara, Y.; Asano, T.; Yasuhara, H. Ebselen in Acute Ischemic Stroke. Stroke 1998, 29, 12–17.
- Otomo, E.; Tohgi, H.; Kogure, K.; Hirai, S.; Takakura, K.; Terashi, A.; Gotoh, F.; Maruyama, S.; Tazaki, Y.; Shinohara, Y.; et al. Effect of a Novel Free Radical Scavenger, Edaravone (MCI-186), on Acute Brain Infarction. Randomized, Placebo-Controlled, Double-Blind Study at Multicenters. Cerebrovasc. Dis. 2003, 15, 222–229.
