Lignin, being a natural aromatic polymer rich in functional hydroxyl groups, has been drawing the interest of academia and industry for its valorization, especially for the development of polymeric materials. Among the different types of polymers that can be derived from lignin, polyurethanes (PUs) are amid the most important ones, especially due to their wide range of applications. Lignin, being a natural aromatic polymer rich in functional hydroxyl groups, has been drawing the interest of academia and industry for its valorization, especially for the development of polymeric materials. Among the different types of polymers that can be derived from lignin, polyurethanes (PUs) are amid the most important ones, especially due to their wide range of applications.
- lignin
- polyols
- polyurethanes
1. Introduction
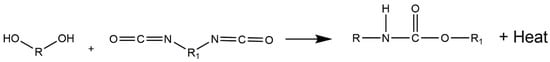
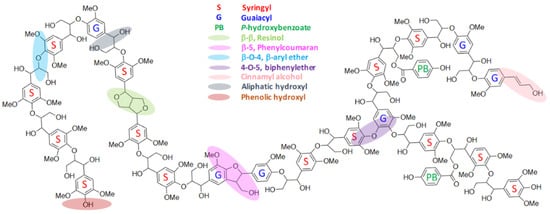
2. Lignin and Its Structural Features
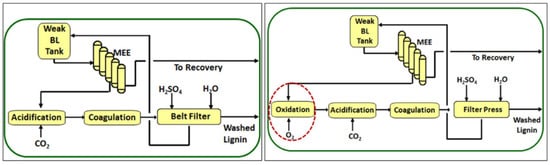
3. Technical Lignins
4. Synthesis of Lignin-Based Polyether Polyols
4.1. Lignin-Based Polyol via Oxyalkylation Reaction
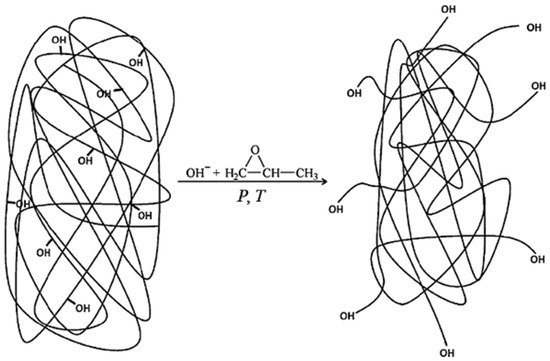
4.2. Lignin-Based Polyol via Liquefaction with a Polyhydric Alcohol
4.3. Quality of Lignin-Based Polyol and Its Characterization
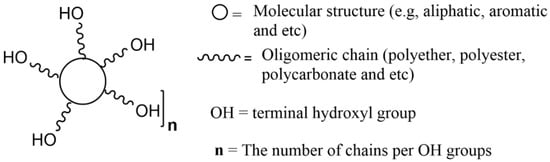
5. Lignin as a Building Block to Synthesize Polyurethanes
The use of lignin in the production of PUs can be carried out in different ways: unmodified, being directly incorporated into polyol formulations, after fractionation, or after chemical modification (in order to make it more reactive), alone, or in combination with other polyols [46,47,102,103,104,105,106,107]. Depending on its interaction with the isocyanate, lignin can act as filler or as reagent, i.e., as polyol, also referred to as cross-linker. Although the use of lignin without any treatment is widely reported, if its OH groups do not react with the isocyanate and become chemically bonded to the PU network, it should not be described as a building block. The direct exploitation of lignin, as a polyol on its own or blended with industrial polyols, is energetically and environmentally advantageous [108], and the ensuing biomass-based PUs are more biodegradable than those derived from petroleum-based polyols. Even though the direct use of lignin as the only polyol can be very appealing, generally, lignin macromonomers have low reactivity towards isocyanate groups yielding products without the desirable performance, and end up acting essentially as fillers [46,109,110].
Recent advances to circumvent the drawbacks of direct incorporation of lignin as polyol in the production of PUs include (1) the use of diols and glycerol as compatibilizer and cross-linker, and (2) lowering the lignin molecular weight using solvent fractionation. Since kraft lignin is the most commonly produced technical lignin, most of the studies are focused on this type of lignin. For example, Haridevan et al. [112] recently evaluated the dispersion and solubility of kraft lignin in different types of polyols at room temperature for the production of polyurethanes based on microscopic, gravimetric, and rheological analyses. This study demonstrated that kraft lignin has different degrees of dispersion in various polyols, depending on the structural characteristics such as solubility parameter, molecular weight, and monomeric unit type. In fact, it was observed that a higher degree of dispersion of kraft lignin was achieved in the lower-molecular-weight diethylene glycol (106.1 g/mol) than in polyethylene glycol (400 g/mol). Although the low dispersibility of lignin in polyols has not yet been solved, systematic studies such as this one are important contributions to increase lignin dispersion. In addition, the performance of PUs can be improved by heating the polyol/lignin dispersions at 120 °C prior to the reaction, which enhances the disaggregation of lignin microparticles, yielding a better lignin dispersion within the polyol system [113,114]. On the other hand, reducing the kraft lignin molecular weight by solvent fractionation can enhance its miscibility and dispersion in the PU matrix, and consequently can improve some properties of the resulting PU such as the mechanical properties [115]. The use of binary organic solvent mixtures such as acetone–methanol [116], aqueous two-phase systems (ATPSs) composed of (NH4)2SO4 and ethanol [117], and the use of ionic liquids [118] are examples of strategies used to reduce the molecular weight of lignin as well as its heterogeneity. However, it is important to take into account that commercializing low-molecular-weight lignin has been economically unfeasible until now.
In addition to PUF, different types of lignins and LBPs have also been used to prepare other forms of Pus, such as adhesives [121,127,128], elastomers [129,130,131], coatings [132,133], and films [125]. Elastomeric PUs are a class of PU materials that have the characteristics of rubber, and their application has increased in recent years due to the high demand for advanced applications such as in biomedicine, shape memory materials, self-healing materials, and gel materials. Mechanical properties such as toughness, tensile strength, and high elongation at break are highly desirable for the production of PU elastomers, and the use of modified or unmodified lignin in the synthesis of PU elastomers has shown that it plays an important role in improving these properties, where a percentage of lignin up to 40 wt.% (based on mass of polyol) did not jeopardize their mechanical properties [134,135,136].
In conclusion, the properties of lignin have a profound impact on the resulting PU product performance, e.g., foams, elastomers, coatings, and films, regardless of the lignin source and isolation process [132,133,134]. Nevertheless, with the exception of a few examples, the exact role of the structural features of lignin during the reaction with isocyanates is hardly discussed. Instead, the mixture of unmodified lignin with other polyols or of LBPs is treated globally as the polyol component of the PU. Yet, with the advancement of fractionation methodologies, a better understanding of the role of structural features of this renewable OH-rich aromatic material will certainly become clearer and bring further insights.
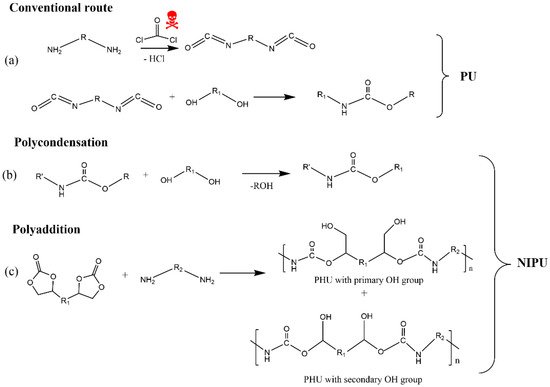
6. Lignin as a Building Block to Synthesize Non-Isocyanate Polyurethane (NIPU)
The industrial synthesis of isocyanates involves the reaction between primary amines and phosgene at high temperatures (100–200 °C). The latter is a highly toxic gas, produced by the reaction between carbon monoxide and chlorine gas. In turn, diisocyanates cause acute adverse health effects, such as irritation of the respiratory tract, eyes, and skin, being a major cause of occupational asthma in workers employed by the polyurethane industry [153,154,155,156,157]. Moreover, the two most widely used isocyanates in the PU industry, MDI (methylene diphenyl diisocyanate) and TDI (toluene diisocyanate), are highly reactive chemicals that bind to DNA and are probably genotoxic [158]. Furthermore, various household PU products, such as mattresses, pillows, cushion packaging, and insulating materials in building construction, among others, exhibit detrimental environmental impact on aquatic life, soil health, plants, and humans due to the presence of toxic components such as isocyanates, flame retardants, and amine-based catalysts [159]. Additionally, some compounds, such as carbon dioxide, carbon monoxide, hydrogen cyanide, acetaldehyde, and methanol, are released when PU products are burned and/or landfilled at their end-life, contributing to the greenhouse effect and having toxic effects on human health [160,161]. All these reported issues led the European Union to adopt the regulation where it was proposed to reduce the content of isocyanate, with the main goal being to ban its use in the future [162].
7. Concludsion
This entry is adapted from the peer-reviewed paper 10.3390/ma15176182
