2. Magnetic Polymer Nanocomposites (MPNs)
Polymers, metals, and ceramics are used as matrix materials for composite materials [
11]. In short, the matrix is the structure that holds the reinforcement material. The most common and commercially available matrix materials used to make polymer composites are polyester resin, epoxy resin, and vinyl ester resin [
12]. Reinforcement materials transmit the loads to the matrix; therefore, they define most of the mechanical characteristics of the material, such as resistance and rigidity, as well as magnetic, optical, and electrical properties of the final composite material, reducing costs [
13]. Composite materials improve the aircraft system by reducing the aircraft’s weight, thereby decreasing the fuel cost per passenger. It is important to note that the three most common composite materials available are reinforced with glass fiber, carbon fiber, and aramid fiber. Currently, MNPs are frequently studied as part of a composite for airplane purposes [
4].
Reinforcement can make up 20–80% of the volume of the composite material, but usually less than 50% [
14]. In the simplest case, the appropriate addition of reinforcement to a polymeric matrix creates a functional material and it can improve its performance, often dramatically, simply by taking advantage of the nanoscale nature and properties of the filler [
15,
16,
17]. Among the nanosized fillers, iron oxides have attracted significant interest, and magnetite (Fe
3O
4) and maghemite (γ-Fe
2O
3) especially have found numerous potential applications in magnetic recording technology, pigments, catalysis, photocatalysis, medical uses, and environmental processes [
18].
MPNs materials constitute a new generation of multifunctional materials that combine the properties of conventional polymers and magnetic materials (ferrimagnetic and/or ferromagnetic particles mixed or embedded in a matrix) are classified as “magneto-polymeric materials” [
19] or also known as ferrogels [
20]. MPNs typically contain nanosized magnetic materials to trigger the response to an external stimulus (i.e., an external static or alternating magnetic field) [
21]. Within ferrogels, elongation, contraction, and curvature can be induced by applying a homogeneous magnetic field [
19].
Ferrites are ceramic-like compounds derived from iron oxides combined with metallic elements. These types of materials are non-conductive and ferrimagnetic. Due to their excellent magnetic and dielectric properties, ferrites are considered one of the best magnetic materials to be used with polymeric matrices in electromagnetic wave absorption applications [
22]. This material’s carbon-based reinforcements are graphite, carbon fibers, carbon nanotubes, and graphene sheets. As a general trend, it is found that increasing the reinforcement content within a matrix increases the electrical conductivity, which is a fundamental factor for shielding and absorbing electromagnetic waves [
23].
The inclusion of nanoparticles of inorganic origin dispersed in a polymeric matrix helps to improve mechanical, magnetic, optical, and even thermal properties [
24]. However, this improvement will largely depend on the type of dispersion, which can be homogeneous or diffuse [
25]. Li et al. modified the surface of poly(vinylidene fluoride-hexafluoropropylene) with BaTiO
3 nanofibers (BT NFs). The results showed that the nanocomposite with 3 vol% BT NFs has an improved discharged energy density of 8.55 J/cm
3 in an applied electric field (300 MV/m). This value is 43% higher than the pristine polymer matrix. This improvement is suitable for aerospace power systems, where, usually, high energy densities are achieved at ultrahigh applied electric fields (>400 MV/m) [
26]. A recent study by Li et al. showed that the polarity of polymer shells on BaTiO
3 nanoparticles has an interesting effect on the dielectric and energy storage of dielectric polymer nanocomposites [
27].
MPNs can be synthesized by embedding magnetic particles into a non-magnetic (polymer) matrix, selected according to the technology used for fabricating the printed magnetic parts (e.g., polymer matrix, curable hydrogel, or solvent) [
28]. To create exceptionally proficient attractive materials, the “doping” of polymer materials with attractive MNPs, made of an inorganic issue (frequently superparamagnetic press oxide Fe
3O
4 or γ-Fe
2O
3, or “delicate” metallic iron, yet additionally “hard” attractive materials, e.g., Ni, Co, FeN, FePd, FePt, etc.), gave off an impression of being a more engaging and effective arrangement [
29]. These classes of material receive significant attention due to their potential in the fields of catalysis, sensor, enzyme immobilization, DNA extraction, drug delivery [
30,
31], bioremediation [
32], and aerospace [
23].
The massive development of polymers has driven their use in various fields such as aerospace, marine, automotive, military, and structural applications. The critical factor for using any composite material is performance and the degree of deterioration it may have. These factors will largely depend on the duration and interaction with your environment. The deterioration of polymeric materials will involve changes in their composition, both chemical and physical, due to the multiple reactions that can occur, including the cleavage of important bonds within the macromolecule. These changes lead to materials with very different characteristics, which are generally worse than the original material. These deteriorated materials will not contribute to the mechanical properties, and their useful life will be gradually diminished. For this reason, some polymers or its compound used outdoors must respond effectively to environmental conditions [
33].
Thermal stability and flame retardancy are among the most important properties that a polymer or composite with aerospace applications must meet. Research in this field indicates that specifically modified epoxy nanocomposites have better flame properties than conventional composite materials [
34]. According to the Kissinger method, the activation energies for the thermo-oxidative degradation process of modified materials are lower than pure epoxy in the first degradation stage. However, in the second thermo-oxidative stage, an inverse behavior is seen. In the same vein, it has been found that carbon nanotubes tend to outperform nanoclays as effective flame-retardant additives if the carbon nanoparticles form a network anchored to the polymer matrix so that the composite material behaves similar to a gel.
Thermal degradation of composite materials depends on the charge of the clay, the composition, and the nature of the ambient gas. A literature review by Leszczynska et al. analyzed the thermal stability of various matrices modified by montmorillonite clay and its influence factors [
35]. Polymers have a hydrophobic character, so the clay must be modified with a surfactant that allows it to interact with the polymer [
36]. Likewise, certain factors were found that influence the thermal stability of nanocomposites, such as the chemical constitution of the modifier, the chemical character of the polar compatibilizers, the natural thermal resistance of the matrix, the composition of the nanofiller, and also the access to oxygen of the material during the heating process. The clay is modified using ammonium salts as surface-active agents. Thus, the quaternary ammonium ion is commonly used to allow the adhesion of the silicate with a polymeric resin [
37].
In general, including clay in a polymeric matrix considerably improves performance since it acts as a superior insulator and a mass transport barrier for the products generated during decomposition. In this sense, clay acts as a thermal barrier, which can be used to improve the thermal stability of the composite and help the formation of carbon during the decomposition process [
3]. It is important to note that the temperature at which volatilization is generated in a nanocomposite is much higher than in its similar scal (microcomposite). Additionally, the thermal oxidation of the polymer undergoes a significant decrease in a nanocomposite that presents a high carbonization performance. This effect can be produced both by a catalytic action due to the presence of the silicate and the sites created by thermal decomposition, and by the barrier effect between both environments [
37].
Studying a polymer’s performance and degradation is vital for the scientific community and the industry since its understanding will increase the composite’s useful life. Polymer degradation is caused by biodegradation, oxidation, mechanical and catalytic degradation, and pyrolysis. Due to their molecular structure, polymers are susceptible to harmful environmental changes. It should be emphasized that due attention has not been paid to the durability and performance of polymeric nanocomposites in terms of their synthesis processes and the evaluation of their mechanical properties [
38].
Regarding the performance of polymeric nanocomposites, several studies have been carried out. The addition of clay to epoxy was found to decrease the curing reactivity of the resin. This involves a process that reduces the crosslinking density of the cured resin and the greater length of the polymer chains that lie between the crosslinking points [
39]. Having longer polymer chains (which are less thermodynamically stable) together with smaller chains produces a composite with a greater tendency to degrade [
40]. On the other hand, the silicate layers turn out to be barrier phases to common gases such as oxygen and nitrogen, thus allowing the material to isolate itself in the presence of these gases and extend its degradation time [
41]. It was found that nanocomposites intercalated with 10 wt.% clay predominate the first mentioned factor, while in exfoliated materials (2 wt.% clay), the second is more important [
23]. For this reason, it can be concluded that exfoliated materials have better barrier properties than intercalated ones [
15].
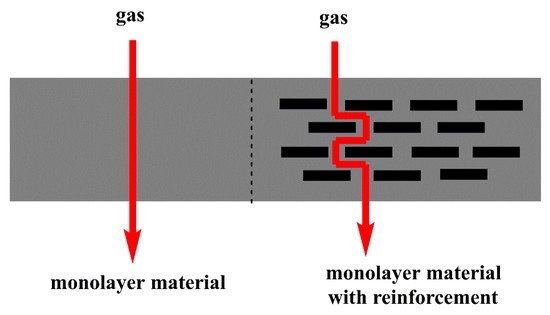
The inclusion of nanoparticles can improve the barrier properties of material since the shape, size, degree of dispersion, and especially type of the particle–polymer interaction affect the transport properties of gases [
42]. The inorganic particles added to a polymeric matrix constitute an impermeable phase; they represent a physical diffusion barrier, making the path that the gases must travel to cross the material longer, as shown in
Figure 2. They can also act as nucleating agents, which affects the polymer crystals’ size and shape and restricts the movement of the chains. Models have been developed to explain and predict particle addition´s effect on permeability. In general, these are based on the effect of the particles on the tortuosity of the membrane since it is considered that the properties and characteristics of the polymeric matrix are not affected by the filling [
43].
Figure 2. Gas diffusion through a matrix with and without nanoparticles.
Regarding the magnetic properties of nanocomposites, it is essential to point out that organic–inorganic interactions add new properties that are impossible to obtain in organic or inorganic materials by themselves and provide a great capacity for transformation. The literature mainly shows two areas of magnetic nanocomposites’ application, as seen in the main nanotechnology initiatives in the United States and the European Community. One of them is the field of biomedicine, and the other one is the development of methods for manufacturing nano-organized structures and their applications and recording devices, sensors, and structures.
Polymeric nanocomposites are good candidates for applying of ferromagnetic nanoparticles on their surface as they have an extraordinary ability to be used as templates and in self-assembly. For example, the ordering can be achieved by self-assembling nanoparticles coated with a surfactant or in situ growth of the particles in a block copolymer as a template [
44].
Functional materials are fundamental ingredients in the design of modern sensors and devices. Some of the many possibilities of combining magnetism with other properties in a polymer nanocomposite are:
-
Magnetic conductive materials: These are useful in manufacturing sensors and devices. They are made up of magnetic nanoparticles in a conductive polymeric matrix. A charge transfer can be established between the surface of the particles and the polymer, so the material acts as an electronic system. Some proposed compositions are magnetite-polyaniline, maghemite-polypyrrole, cobalt ferrite-polypyrrole, and various metal-polymer combinations [
45,
46]
-
Transparent magnetic materials: As magnetic oxides are considerably more transparent to visible light than nanoparticles, magnetic nanocomposites can be made with reasonable transparency and greater magnetization, by more than an order of magnitude, than stronger ones such as transparent magnets.
3. Synthesis of Magnetic Polymer Nanocomposites
3.1. Molding
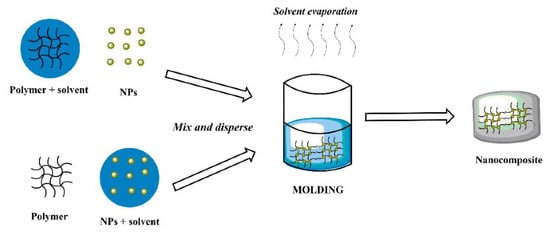
Molding is a conceptually simple form of transferring patterns by soft lithography [
52]. It is a widely used fabrication process of MNCs that is accomplished by mixing magnetic fillers and polymeric precursors thoroughly and curing them to form specific shapes or structures in molds [
24] (
Figure 3). There are several types of molding processes, such as injection [
53], resin transfer [
54], and compression molding techniques [
55], that practically use a mold that is completely filled under pressure and temperatures. The mold walls are heated to a temperature above the melting point of the mold material allowing a faster flow of material through the cavities [
56].
Figure 3. Schematic representation of molding method. Reprinted with permission from ref. [
57], MDPI, 2022.
3.2. Coprecipitation
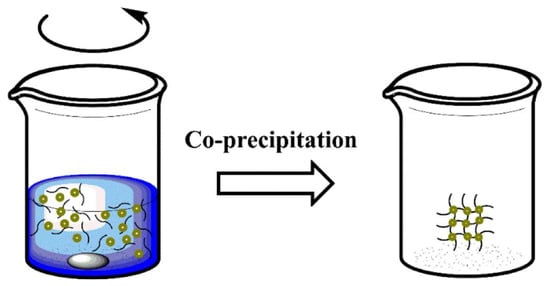
Coprecipitation is a very facile and convenient way to synthesize iron oxide nanoparticles (either Fe
3O
4 or γ-Fe
2O
3) from aqueous Fe
2+/Fe
3+ salt solutions by the addition of a base under an inert atmosphere at room temperature or at elevated temperature [
58] (
Figure 4). It is highly important to study and control the reaction parameters (e.g., type of metal cation precursors, molar ratio between M(II) and Fe(III) cations, reaction temperature, pH value, and type/concentration of alkaline agent) [
59]. The reactant undergoes precipitation (supersaturation of the metallic oxide in the solution) to reach NPs of a particular size [
60]. MNCs have been studied by the coprecipitation method, which is generally synthesized from salt species such as Fe
2+ and Fe
3+ in an alkali solution and under non-oxidizing conditions [
61].
Figure 4. Schematic representation of co-precipitation method. Reprinted with permission from ref. [
57], MDPI, 2022.
Mehra et al. used an in situ co-precipitation method to achieve a homogeneous melamine-cyanurate (MC) distribution in a polymer matrix. As a result of this incorporation, 65% enhancement of thermal conductivity was achieved, offering a new strategy for the development of new thermally conductive materials [
62].
3.3. In Situ Precipitation
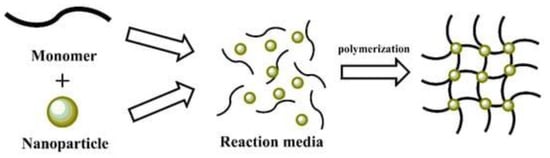
The in situ precipitation method is widely used and reported in the literature to synthesize MNPs based on their application in many fields [
49]. This method involves processes in either a solvent-free system (i.e., a bulk phase) or in a solvent-based system (including an aqueous phase) that can be purely solvent, emulsion, or suspension-based [
63], which is combined with magnetic nanofillers [
64], resulting in MNCs as
Figure 5 shows. This simple and straightforward method loads MNPs into a polymeric matrix because this method involves the inclusion of nanoparticles into a polymer matrix in the presence of a precipitation medium [
65]. The selective precipitation of small amounts of inorganic nanoparticles within the porous matrix reduces the accessible pore volume [
66].
Figure 5. Schematic representation of in situ polymerization method. Reprinted with permission from ref. [
57], MDPI, 2022.
Practically, magnetic composites can be synthesized by embedding magnetic particles into a non-magnetic matrix [
67]. Konwar et al. reported the synthesis of MCs using the in situ polymerization method. Firstly, the chitosan hydrogel was prepared using glutaraldehyde as a cross-linker, then the MNPs were placed to form inside the hydrogel matrix via the co-precipitation process, resulting in obtaining MNCs [
68]. Wang et al. carried out fabrication via the in situ growth of nano Fe
3O
4 on the polydopamine (PDA)-functionalized MoS
2 nanosheets to generate solar steam. The magnetic MoS
2 nanosheets not only showed the long-term dispersion in an aqueous solution due to the introduction of hydrophilic PDA but also exhibited fast and effective separation from the aqueous solution with the help of the decorating nano Fe
3O
4, which benefit the continuously efficient solar steam generation and its good recyclability [
69].
3.4. Blending
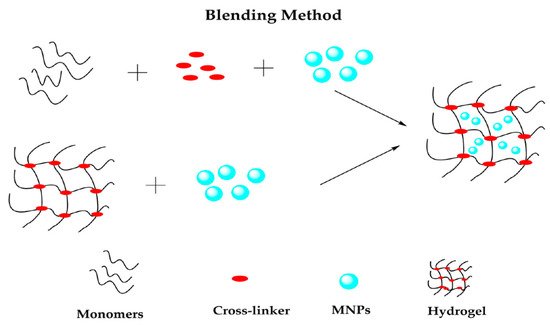
The blending method consists of the simple mixture of MNPs and the hydrogel precursor solution and the subsequent polymer cross-linking, producing hybrid magnetic loaded hydrogel networks [
70]. In other words, this technique consists of the physical encapsulation of MNPs into a polymeric matrix [
67] in two ways, as
Figure 6 shows. Firstly, preformed MNPs are placed into the aqueous polymer solution, causing polymer chains to cross-link and encapsulate the MNPs, while the second method corresponds to MNPs and network hydrogel being made separately, and afterward, the MNPs are trapped in the network by physical interactions [
50]. Such encapsulation of NPs helps stabilize NPs by preventing them from agglomerating [
71].
Figure 6. Schematic representation of blending method. Reprinted with permission from ref. [
50], MDPI, 2022.
Melt blending is a more adaptable technique specifically for thermoplastic NCs [
72]. This technique is simple, environmentally friendly (since no solvent is needed), cost-effective, and best for mass production [
73], in the presence of an inert gas such as argon, nitrogen, or neon [
74]. This kind of mixing ensures that the NPs are exfoliated in the polymer matrix by fixing the MNPs within the polymer matrix just as they are in water [
75,
76].
Thu et al. reported preliminary results on preparing magnetic nanocomposites based on acrylonitrile butadiene styrene (ABS) and nickel nanorods (NiNRs), which were used as magnetic components. The magnetic nanocomposites were prepared by first incorporating NiNRs into the ABS matrix via a process of solution blending and then evaporation of the solvent used [
77].
3.5. Grafting Methods
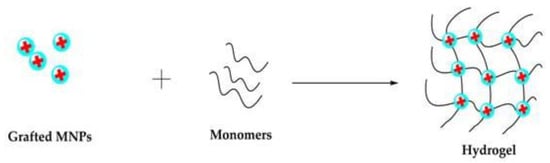
In “grafting to” techniques, polymer chains modified with anchoring groups are used to bind to the particle surface. Likewise, the MNPs surfaces are modified with suitable functional groups that act as cross-linkers in the presence of the hydrogel precursor, allowing the formation of hydrogels with the MNPs covalently bonded to the polymeric networks [
78].
Figure 7 illustrates the grafting of MNPs with monomers, forming an MNC. The magnetic polymeric matrix produced by the grafting method shows higher NP dispersion stability due to the covalent coupling [
16].
Figure 7. Schematic representation of grafting method. Reprinted with permission from ref. [
50], MDPI, 2022.
In the grafting-onto method, the polymer backbone and the polymer side chains are prepared separately and coupled afterwards [
79]. In this case, grafting does not involve a chain reaction [
80]. Gamma radiation has been successfully developed to graft filler onto polymeric materials [
81]. The grafting method is based on the surface modification of MNPs with functional groups to interact with polymer chains covalently [
82].
Hu et al. designed a magnetic hydrogel made from non-toxic polyacrylamide (PAAm) hydrogel and 3-(trimethoxysilyl)propyl methacrylate-coated Fe
3O
4 via the grafting-onto approach. This magnetic hydrogel not only offers a relatively high modulus and toughness compared to the pure hydrogel but also responds to the magnetic field rapidly because of high magnetic particle content [
50,
83]. Jia et al. grafted aramid fibers, 3-aminopropyltriethoxysilane (APS), onto the epoxy matrix. The reinforced aramid fiber composite increased by 51.03%, from 36.33 to 54.87 MPa, after the modification, providing remarkable news for potential applications of aramid composites in aircraft and aerospace [
84]. Islam et al. developed a novel method to graft carbon nanotubes (CNTs) onto carbon fiber (CF) via ester linkage, forming a CNT-CF hierarchical reinforcing structure. Results show that the fibrous film of CNT-CF exhibits a specific capacitance superior to pristine CF (3.5 times greater), indicating a potential application as a supercapacitor with enhanced performance [
85]. In this sense, Zhao et al. scattered highly conductive carbon nanotubes into a PU matrix to produce nanocomposites for electrostatic dissipation. Dispersion of these carbon nanotubes includes the “grafting onto” method in its strategy. An addition of 1 wt.% of these components improves the tensile strength and modulus compared to pristine PU film, making it a flexible nanocomposite with low surface resistivity for aerospace coating applications [
86].
All synthetic methods display advantages and disadvantages in manufacturing nanocomposites. Table 1 shows a comparison between those reviewed, covering a short description of the methods, their advantages and disadvantages, and a reference related to the topic. It is interesting to note that differences between them lie in their procedure, their obtained properties, their environmental impact, and their cost, which are key factors in obtaining nanocomposites.
Table 1. Summary of synthetic methods for polymer nanocomposites with their advantages and disadvantages.
| Synthetic Method |
Brief
Description |
Advantages |
Disadvantages |
References |
| Molding |
A polymeric stamp is placed in contact with a precursor of a solid material |
|
|
[52] |
| Co-precipitation |
Reducing a mixture of metallic ions using a basic solution at low temperature and in an inert atmosphere |
|
-
Properties of obtained particles (size, shape, and composition) are highly dependent on reaction parameters
-
Need to add low molecular weight surfactants to stabilize obtained nanoparticles
|
[58] |
| In situ precipitation |
Nanoparticles dispersed in a monomer or monomer solution and polymerization under standard techniques |
|
|
|
| Blending |
Polymer melted with a desired amount of filler in presence of an inert gas and heat |
|
|
[64] |
| Grafting |
Dispersion of nanoparticles along the surface polymer matrix initiated by radical polymerization |
-
Covalently grafted filler on the solid surface
-
Good control over the polymer molecular weight
-
Application in a wide range of monomers, obtaining nanocomposites with different functionalities
-
Good dispersion of filler
|
|
[87] |