Please note this is an old version of this entry, which may differ significantly from the current revision.
Antibody secreting cells (ASCs) constitute a variable fraction of tumor-infiltrating B cells in most solid tumors, and they produce tumor-specific antibodies that can drive distinct immune responses depending on their isotypes and specificities.
- breast cancer
- ovarian cancer
- antibody-secreting cells
1. Introduction
B cells and antibody secreting cells (ASC) are critical effectors of the adaptive immune system. Although their importance in infectious disease and vaccination is well-recognized, their roles in cancer and response/resistance to immunotherapies have been overlooked for a long time in favor of T cells. B cell infiltration has been documented early on in many solid tumors using conventional IHC with markers such as CD19 and CD20. B cells are rarely found alone and are usually closely associated with T cells and myeloid cells, and can account for up to 60% of the immune infiltrate in some patients [1][2][3][4][5]. The diversity of tumor-infiltrating (Ti) B cells can be captured using flow cytometry and medium- to high-dimension in situ tumor tissue staining by combining B cell subset-specific markers, computational analyses of bulk RNAseq data using gene signatures or deconvolution tools, single-cell RNAseq, and, more recently, spatial transcriptomics. Ti B cells can encompass naïve and activated B cells, several populations of antigen-experienced memory B cells and ASCs, and, occasionally, germinal center (GC) like B cells [1][6][7]. The presence of this continuum of B cell differentiation stages usually typifies so-called immune hot tumors where B cells are functionally organized with other immune cells in so-called tertiary lymphoid structures (TLS), resembling secondary lymphoid organs, with segregated B cell and T cell zones, mature dendritic cells (DC), and specialized blood vessels. Such structures can initiate and/or amplify powerful in situ cellular and antibody responses, and are associated with better patient prognosis and response to immune therapies [8]. In addition, B cells producing suppressive cytokines such as IL10, e.g., so-called regulatory B cells (Bregs), were also identified in the tumor microenvironment (TME) in certain tumor types and were demonstrated to dampen anti-tumor immunity [9][10][11][12][13][14][15][16]. Ti-ASCs consist of plasmablasts and plasma cells, and constitute a multifunctional B cell subset that can impact tumor cells and cells from the TME in multiple ways. Indeed, each ASC can not only produce a huge quantity of monoclonal immunoglobulins of a unique isotype that recognize a specific (tumor) antigen (Ag), but can also exert noncanonical functions through the release of pro- or anti-inflammatory cytokines and/or the expression of immune checkpoint ligands able to modulate antitumor immunity.
2. ASCs Infiltrate Breast and Ovarian Tumors
Antibody-secreting cells, identified using morphological features or by expression of CD38 or CD138 through immunohistochemistry or immunofluorescence, can be detected in approximately 30% of BC and high-grade serous OC (range from 12% to 70% according to studies) and their density within the tumor infiltrate can vary widely from patient to patient [3][7][17][18][19]. Such variations can be explained by several factors, including (i) the type of analyzed tissue (e.g., ASCs were found to be increased in ovaries and metastases compared to fallopian tubes [20]), (ii) the histology and molecular classification of the tumor (e.g., ASC infiltration is typically higher in medullary [17][19], HER2+ and triple-negative BC compared to ER+ BC [21]), (iii) tumor-specific characteristics, (e.g., expression of tumor-associated Ags as described in OC where ASC infiltration was positively associated with the expression of the cancer–testis antigens NY-ESO-1, MAGEA1 and CTAG2 [2]), and iv) lack of a truly specific ASC marker (CD138, often used as a biomarker to identify ASC, can be expressed by other cells and is poorly detectable on immature ASC, e.g., plasmablasts (PBs), which infiltrate up to 80% of tumors [18][22]). However, the degree of ASC infiltration does not seem to vary with tumor mutation load, BRCA1/2 status and P53 mutations [7]. Several general features are associated with Ti-ASCs. They preferentially localize in the tumor stroma [23][24] in the vicinity of CD8+ and CD4+ T cells, B cells, and TLSs [7][17][25]. In most cancer types, Ti-ASCs produce mostly IgG antibodies (Abs), although this may vary depending on the type of tissue (nonmucosal vs. mucosal origin of the cancer tissue) [7][19][26][27][28][29][30][31][32][33][34]. Recently, Biswas et al., reported intriguing results showing that ASCs infiltrating OC were mainly producing IgAs [35], contrasting with other studies indicating a dominance of IgG-expressing cells [7][26]. The reasons for these discrepancies remain currently unknown but could be linked to the use of tissue from different anatomical locations, to different treatments received by patients, and/or to the possible presence of adjacent normal mucosal tissue.
3. Antibodies in Breast and Ovarian Cancer Patients
Antibodies directed against a broad array of tumor- and self-Ag are frequently detected in the serum and the tumor microenvironments (TME) of cancer patients. These Ags include aberrantly- and over-expressed proteins, oncoviral and intracellular proteins, endogenous retroelements, and occasionally neoantigens derived from tumor mutations. Early approaches to studying the repertoire of antibodies in cancer patients include serological expression cloning (SEREX) [36] and phage display [37] methods. For example, NY-ESO-1, a well-known cancer testis antigen aberrantly expressed in various solid tumors, including BC and OC, was discovered by SEREX based on its capacity to induce potent humoral response in cancer patients [38]. The magnitude of such antibody responses varies greatly according to the cancer type and from patient to patient. Profiling of serum antibody specificities using microarrays assembling more than 8000 proteins has indeed revealed that more than 200 Ags were targeted by IgGs in OC, compared to less than 30 in pancreatic cancers, revealing their difference of immunogenicity [39]. Levels of circulating antibodies to tumor and self-Ags can have some prognostic and diagnostic value. For instance, antibodies against mucin 1 (MUC1) can be detected in the serums of patients at early stages of BC and OC and serve as a marker of good prognosis [40][41], whereas anti-p53 antibodies have been associated with an unfavorable outcome in BC [42][43]. Few studies so far have investigated other antibody isotypes than IgG. Interestingly, anticalreticulin IgGs and IgAs were reported in various solid cancers, and IgAs were more often associated with breast tumor metastasis than were IgGs [44], suggesting differential roles of the two Ab isotypes. In BC, Ab levels to different sets of Ag have been proposed for early detection of the disease, including the in situ stage [45]. Such diagnostic value of circulating Ab is best exemplified in paraneoplastic neurological diseases, which are rare autoimmune diseases that develop in a fraction of cancer patients. Elevated levels of Ab to so-called onconeural-Ag, e.g., Ags expressed physiologically by neuronal cells and aberrantly by tumor cells, are not only used for the diagnosis of the neurological disease but can also predict the type and tissue location of tumors, which can be indolent, causing it to be difficult to diagnose in a significant proportion of patients [46][47][48].
Circulating tumor-specific antibodies likely originate from plasma cells (PCs) residing in classical niches, such as the bone marrow and spleen, but also from PCs present in the TME. Profiling of supernatants of B cells isolated from lung tumors and activated in vitro and of supernatants obtained during dissociation of breast tumors with focused sets of Ag, revealed IgGs and IgAs against one or several tumor Ag in each patient, indicating local production of tumor-specific Ab in the TME [49][50]. Interestingly, while most antibody reactivities were detected in both the serum and tumor, some were restricted to a single compartment, indicating that systemic and local Ab responses in cancer patients are partly disconnected. It is indeed likely that the repertoire of Ab in the TME is more restricted and focused on tumor Ag, especially in tumors with TLS, which promote the Ag-driven expansion of B cells and their differentiation into PCs [51][52].
4. Origin of Tumor-Infiltrating ASCs
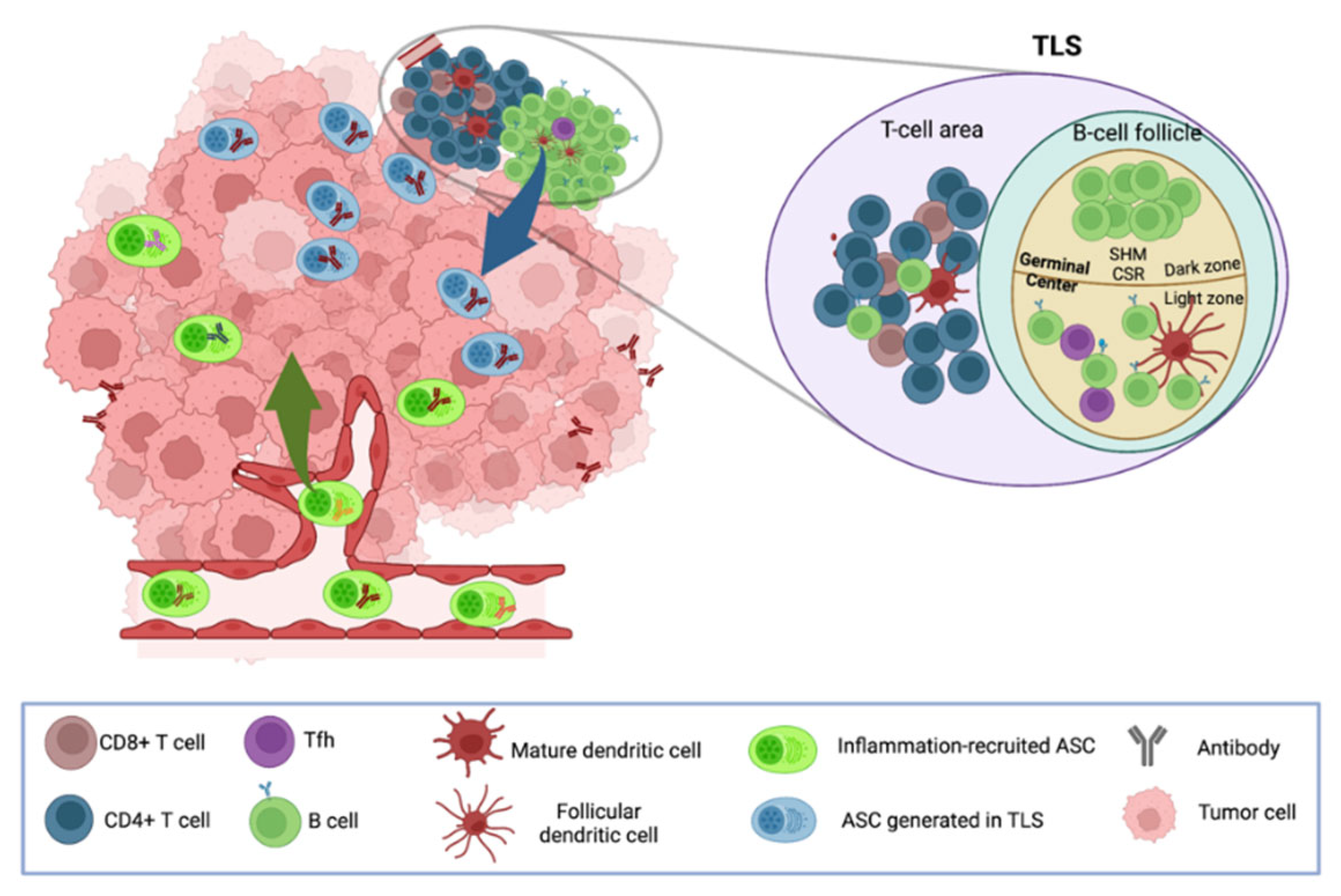
There is increasing evidence that Ti-ASCs consist not only of cells differentiated outside the TME and attracted from blood to the tumor bed, but also of cells that have locally differentiated from recruited naive and/or memory B cells. It is now well established that PBs generated in secondary lymphoid organs (SLO) can acquire different chemokine receptors, like CXCR4, CCR9, and CCR10, allowing their migration to the bone marrow to constitute a contingent of long-lived PCs and to different effector sites including mucosal tissues. Studies in vaccination models revealed that PBs can also express CXCR3 allowing their migration to inflammatory sites in response to gradients of the inflammatory chemokines CXCL9, CXCL10, or CXCL11 [53]. In OC, Kroeger et al., indeed showed that Ti-IgG+ ASC universally expressed CXCR3, suggesting that this chemokine receptor may contribute to their recruitment [7].
With the discovery of TLSs in certain tumors, it is now admitted that inflammation-driven recruitment from blood does not account for all ASCs present in the TME. TLSs can develop in chronic inflammatory sites and are organized similarly to SLOs, with B cell follicles adjacent to T cell-rich areas that contain mature DCs and high endothelial venules. They are associated with better patient prognoses in many solid cancers including BC and OC [54][55][56][57]. Numerous evidence support that TLSs are sites where B cells differentiate into ASCs in response to local presentation of tumor Ags. Indeed, in many epithelial tumors, virtually all B-cell differentiation stages, e.g., naïve B cells, activated B cells, germinal center (GC) B cells, memory B cells, PBs, and terminally differentiated ASC (PC), have been identified by flow cytometry and bulk or single transcriptome profiling [1][22][35][58], arguing for an ongoing local humoral immune response in certain tumors. In line with this, TLSs can harbor B cell follicles with GC containing B cells expressing Ki67 and AID, revealing ongoing Ag-driven expansion, somatic hypermutation (SHM), and class-switch recombination [1][49][51][59]. In addition, a strong associations between the presence of TLSs and elevated ASC numbers have been reported in various solid cancers [7].
Examination of the immunoglobulin repertoires of B cells and ASCs from TLS-positive tumors revealed a more oligoclonal response compared to tumors with an unstructured immune infiltrate [7][52][60][61]. Using spatial BCR profiling, a recent study in renal carcinoma revealed clonal selection and expansion in TLS areas and detected the presence of fully mature ASC clonotypes at distance from TLSs, indicating that SHM occurs in TLSs and that mutated ASCs disseminate throughout the tumors [51]. Researchers' own study in ovarian tumors from patients with paraneoplastic cerebellar degeneration, a rare autoimmune neurological disease associated to cancer, revealed tumor Ag deposits in B cell follicles of TLSs [62]. TLSs may thus allow emergence of clonally expanded tumor Ag-specific ASCs. The latter have been recently detected in several cancers, including human papillomavirus (HPV) + head and neck [59] and ovarian [61] cancers. Mazor et al., showed that ASCs that infiltrate ovarian tumors were mutated, clonally expanded, and produced antibodies reacting against metalloproteinase (MMP) 14—an autoAg that is overexpressed in ovarian tumor cells—and able to bind to the surface of tumor cells [61]. Moreover, Meylan and collaborators observed higher proportions of IgG-labeled apoptotic tumor cells in TLS+ tumors compared to TLS− tumors [51], indicating that TLS sustain the production of antibodies reacting with tumor cells and possibly leading to their elimination.
Isotype switching to both IgGs and IgAs can occur in tumors [26][63][64]. It requires two signals: CD40L provided by T follicular helper (Tfh) cells and specific cytokines (produced by Tfh or surrounding cells), which dictate the nature of the switched isotype [65][66]. Three Tfh subsets can be distinguished according to their chemokine receptor and cytokine profiles, namely CXCR3+CCR6− IFNγ-producing Tfh1, CXCR3−CCR6− IL-4/IL-13-producing Tfh2, and CXCR3−CCR6+ IL-17/IL-22-producing Tfh17 cells [67]. In breast tumors, Tfhs are detected predominantly within fully developed TLSs, mostly consist of Tfh1 cells, and can trigger IgG and IgA production by B cells in vitro by a process involving CD40-CD40L interaction [68]. Therefore, it is more likely that the type of intratumorally produced cytokines largely dictates the class/subclass of antibodies expressed by newly differentiated ASCs. Karagiannis and colleagues showed in melanoma tissues that the presence of IgG4 ASC coincides with high levels of IL4 and IL10, two cytokines promoting Th2 polarization and isotype switching toward IgG4s [69][70][71]. Another study in a mouse model of prostate cancer demonstrated that TGF-βR signaling in B cells is mandatory for the induction of IgA PCs with immunosuppressive properties [9]. It can therefore reasonably be supposed that the Ab isotype expressed by locally generated ASCs largely depends on the functional profile of Tfh cells and reflects the cytokine TME.
Altogether, researchers current knowledge indicates that Ti-ASCs may contain cells recruited from the circulation—possibly including some tumor Ag-specific cells generated in SLO—and cells that have differentiated locally in TLS from naïve and/or memory B cells and that are thus likely enriched in tumor Ag-specific cells (Figure 1). A recent study further supports this hypothesis by showing that tumors from patients with HPV-positive head and neck cancers were infiltrated by HPV-specific ASCs with minimal bystander recruitment of influenza-specific ASCs [59].

Figure 1. Possible origins of tumor-infiltrating ASCs. Circulating polyclonal ASCs can be recruited to the tumor site in response to inflammation, whereas oligoclonal ASCs can be locally generated from naïve and/or memory B cells in TLS in response to stimulation by Ags, including tumor Ags.
5. ASC and Antibodies Influence Cancer Patient Survival and Response to Immunotherapies
Numerous studies have evaluated the association between tumor infiltration by ASCs at the time of diagnosis and patient survival after surgery (Table 1). Overall, ASCs are usually associated with a better prognosis that is manifested by increased disease-free and/or overall patient survival. This positive correlation is reinforced in case of tumor co-infiltration by CD8+ T cells [7] and when tumor cells are coated with endogenous immunoglobulins, a situation that is usually accompanied by increased intra-epithelial T cells [35]. Nonetheless, a minority of studies reported an association with a poor prognosis [17][20][72][73]. These discordant results may be linked to the phenotypic and functional heterogeneity of the tumors ASCs infiltrate, as well as to methodological issues linked to the detection of ASCs, which range from a simple morphological identification, IHC/IF analysis with different markers like CD138 and IRF4, to computational analysis of ASC gene signature score from tumor bulk RNA-seq data using diverse algorithms. The use of CD138 as a marker of PC should indeed take into account that other cells, including epithelial cells, can express this cluster of differentiation and could bias prognosis studies [74]. Combining this marker with a morphological identification of PCs or with another ASC marker such as the Ig Kappa light chain (IGKC) should therefore be privileged [20][75][76]. Another possibility would be that the prognosis impact of ASC may vary with their state of maturation, as CD138 is upregulated during ASC maturation into PC but is poorly expressed at the PB stage [77]. The respective impact of PB versus PC in cancer remains so far largely unexplored.
Table 1. ASC impact on patient prognosis. Studies reporting a positive impact are highlighted in blue, while those documenting a negative impact are highlighted in salmon.
| Author/Year | Histological Tumor Type | Number of Patients | Identification of ASC | Prognosis | Reference |
|---|---|---|---|---|---|
| Kroeger et al., 2016 | HGSOC | 30 | CD20−CD38+CD138+cytosolicCD79a+ IHC CD19+IgD−CD38+ Flow Cytometry TNFRSF17/IGJ PC gene signature |
Good | [7] |
| Lundgren et al., 2016 | OC | 209 | CD138 IHC IGKC gene expression |
Poor Neutral |
[20] |
| Yang et al., 2021 | HGSOC | 351 | Gene signature (CIBERSORT) | Poor | [73] |
| Biswas et al., 2021 | HGSOC | 534 | CD19+CD138+ (multiplex IHC) Internalized IgA in tumor cells |
Good (total area and epithelial tumor islets) Good |
[35] |
| Schmidt et al., 2012 | BC OC |
1810 426 |
IGKC expression | Good Neutral |
[76] |
| Iglesia et al., 2014 | BC OC |
728 266 |
IgG cluster | Good (nonluminal BC) Good (mesenchymal and immunoreactive molecular subtypes) |
[78] |
| Gentles et al., 2015 | Pan-cancer | 796 BC 1127 OC |
Plasma cell gene signature (Cibersort) | Good (BC) Neutral (OC) |
[79] |
| Ridolfi et al., 1977 | Infiltrating ductal carcinoma | 192 | Morphological identification on hematoxylin and eosin-stained slides |
Neutral (Medullary carcinoma) Good (others) |
[80] |
| Yeong et al., 2018 | Triple-negative BC | 269 | intratumoral CD38+ IHC stromal CD38+ IHC |
Good Neutral |
[25] |
| Mohammed et al., 2012 | Invasive ductal breast cancer | 468 | CD138+ IHC and morphological identification (H&E) | Poor | [17] |
| Miligy et al., 2017 | Invasive BC | 44 | CD138+ IHC | Neutral | [23] |
| Kuroda et al., 2021 | TNBC | 114 | Stromal CD38+ IHC Intratumoral CD38+ IHC, stromal and intratumoral CD138+ IHC |
Good Neutral |
[81] |
| Fan et al., 2011 | BC | 550 | IGG gene cluster expression | Good | [82] |
| Harris et al., 2021 | TNBC | 69 | Plasma cell signature (Cibersort) | Neutral | [83] |
| Wei et al., 2016 | BC | 92 | Morphological identification (typical “cartwheel” nucleus) | Poor | [72] |
Limited information is so far available regarding the impact of ASCs according to the Ab isotypes they produce. Most studies reported in the literature have analyzed the relation between the relative proportion in the TME of Ig subtypes and patient survival, assuming that Ig detected in the TME, or at the RNA or protein levels, are produced by Ti-B cells. Overall, in most cancers, including melanoma [63][84], lung cancer [85], bladder cancer [86], and prostate cancer [9], a high expression of IgG1 is associated with longer survival, whereas IgG4 and IgA expressions correlate with a negative outcome. However, IgAs were recently reported in OC as associated with a positive impact by impairing tumor growth [35]. In addition, in BC, it was shown that NY-ESO-1 more frequently elicited IgG response, which was associated with poorer prognosis, albeit IgG subclasses were not identified [50]. Moreover, it is difficult to interpret these data as NY-ESO-1, per se, is associated with shorter survival and this result may only highlight the cancer aggressiveness [87]. These results highlight the need to deeply characterize the diversity of ASC in the TME to better appreciate their impact on anti-tumor immunity and patient outcome.
Beyond their usual association with increased patient survival following surgery and chemotherapy, ASCs were recently shown to be also associated with a better response to the checkpoint blockade. By comparing the transcriptome of tumors from patients treated with nivolumab (anti-PD-1) +/− ipilimumab (anti-CTLA4), Helmink et al., documented significantly higher expression of ASC-related genes MZB1, JCHAIN, and Immunoglobulin Lambda Like polypeptide 5 (IGLL5) in patients that responded to treatment compared to nonresponsive patients [6]. Patil et al., showed that PCs, identified with a transcriptional signature including MZB1, DERL3, TNFRSF17, JSRP1, SLAMF7, and immunoglobulin genes, predicted a better overall survival to atezolizumab (anti-PD-L1) in nonsquamous cell lung cancer independently of intra-tumoral CD8+ T cells and PD-L1 expression [88]. In addition, Meylan et al., showed in clear cell renal cancers that patients with a high frequency of IgG-labeled tumor cells had a high response rate to nivolumab +/− ipilimumab and prolonged PFS [51]. The predictive impact of ASCs on immune checkpoint inhibitors may be explained in part by the activation of Tfhs, which strongly express PD1 leading to B cell activation and antitumor immune response, as suggested in murine models [89][90].
This entry is adapted from the peer-reviewed paper 10.3390/cancers14194800
References
- Garaud, S.; Buisseret, L.; Solinas, C.; Gu-Trantien, C.; De Wind, A.; Van den Eynden, G.; Naveaux, C.; Lodewyckx, J.-N.; Boisson, A.; Duvillier, H.; et al. Tumor-infiltrating B cells signal functional humoral immune responses in breast cancer. JCI Insight 2019, 4, e129641.
- Marsigliante, S.; Biscozzo, L.; Marra, A.; Nicolardi, G.; Leo, G.; Lobreglio, G.B.; Storelli, C. Computerised counting of tumour infiltrating lymphocytes in 90 breast cancer specimens. Cancer Lett. 1999, 9, 33–41.
- Milne, K.; Köbel, M.; Kalloger, S.E.; Barnes, R.O.; Gao, D.; Gilks, C.B.; Watson, P.H.; Nelson, B.H. Systematic Analysis of Immune Infiltrates in High-Grade Serous Ovarian Cancer Reveals CD20, FoxP3 and TIA-1 as Positive Prognostic Factors. PLoS ONE 2009, 4, e6412.
- Chin, Y.; Janseens, J.; Vandepitte, J.; Vandenbrande, J.; Opdebeek, L.; Raus, J. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 1992, 12, 1463–1466.
- Buisseret, L.; Garaud, S.; de Wind, A.; Van den Eynden, G.; Boisson, A.; Solinas, C.; Gu-Trantien, C.; Naveaux, C.; Lodewyckx, J.-N.; Duvillier, H.; et al. Tumor-infiltrating lymphocyte composition, organization and PD-1/ PD-L1 expression are linked in breast cancer. Oncoimmunology 2017, 6, e1257452.
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555.
- Kroeger, D.R.; Milne, K.; Nelson, B.H. Tumor-Infiltrating Plasma Cells Are Associated with Tertiary Lymphoid Structures, Cytolytic T-Cell Responses, and Superior Prognosis in Ovarian Cancer. Clin. Cancer Res. 2016, 22, 3005–3015.
- Roumenina, L.T.; Daugan, M.V.; Petitprez, F.; Sautès-Fridman, C.; Fridman, W.H. Context-dependent roles of complement in cancer. Nat. Rev. Cancer 2019, 19, 698–715.
- Shalapour, S.; Font-Burgada, J.; Di Caro, G.; Zhong, Z.; Sanchez-Lopez, E.; Dhar, D.; Willimsky, G.; Ammirante, M.; Strasner, A.; Hansel, D.E.; et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature 2015, 521, 94–98.
- Shalapour, S.; Lin, X.-J.; Bastian, I.N.; Brain, J.; Burt, A.D.; Aksenov, A.A.; Vrbanac, A.F.; Li, W.; Perkins, A.; Matsutani, T.; et al. Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity. Nature 2017, 551, 340–345.
- Machado-Santos, J.; Saji, E.; Tröscher, A.R.; Paunovic, M.; Liblau, R.; Gabriely, G.; Bien, C.G.; Bauer, J.; Lassmann, H. The compartmentalized inflammatory response in the multiple sclerosis brain is composed of tissue-resident CD8+ T lymphocytes and B cells. Brain 2018, 141, 2066–2082.
- Beissert, S.; Hosoi, J.; Grabbe, S.; Asahina, A.; Granstein, R.D. IL-10 inhibits tumor antigen presentation by epidermal antigen-presenting cells. J. Immunol. 1995, 154, 1280–1286.
- Yue, F.Y.; Dummer, R.; Geertsen, R.; Hofbauer, G.; Laine, E.; Manolio, S.; Burg, G. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. Int. J. Cancer 1997, 71, 630–637.
- Cai, C.; Zhang, J.; Li, M.; Wu, Z.-J.; Song, K.H.; Zhan, T.W.; Wang, L.-H.; Sun, Y.-H. Interleukin 10-expressing B cells inhibit tumor-infiltrating T cell function and correlate with T cell Tim-3 expression in renal cell carcinoma. Tumor Biol. 2016, 37, 8209–8218.
- Neves, P.; Lampropoulou, V.; Calderon-Gomez, E.; Roch, T.; Stervbo, U.; Shen, P.; Kühl, A.A.; Loddenkemper, C.; Haury, M.; Nedospasov, S.A.; et al. Signaling via the MyD88 adaptor protein in B cells suppresses protective immunity during Salmonella typhimurium infection. Immunity 2010, 33, 777–790.
- Fillatreau, S.; Sweenie, C.H.; McGeachy, M.J.; Gray, D.; Anderton, S.M. B cells regulate autoimmunity by provision of IL-10. Nat. Immunol. 2002, 3, 944–950.
- Mohammed, Z.M.A.; Going, J.J.; Edwards, J.; Elsberger, B.; Doughty, J.C.; McMillan, D.C. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br. J. Cancer 2012, 107, 864–873.
- Nielsen, J.S.; Sahota, R.A.; Milne, K.; Kost, S.E.; Nesslinger, N.J.; Watson, P.H.; Nelson, B.H. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+ T cells promote favorable prognosis in ovarian cancer. Clin. Cancer Res. 2012, 18, 3281–3292.
- Wang, Y.; Ylera, F.; Boston, M.; Kang, S.-G.; Kutok, J.L.; Klein-Szanto, A.J.P.; Junghans, R.P. Focused antibody response in plasma cell-infiltrated non-medullary (NOS) breast cancers. Breast Cancer Res. Treat. 2007, 104, 129–144.
- Lundgren, S.; Berntsson, J.; Nodin, B.; Micke, P.; Jirström, K. Prognostic impact of tumour-associated B cells and plasma cells in epithelial ovarian cancer. J. Ovarian Res. 2016, 9, 21.
- Pal, B.; Chen, Y.; Vaillant, F.; Capaldo, B.D.; Joyce, R.; Song, X.; Bryant, V.L.; Penington, J.S.; Di Stefano, L.; Ribera, N.T.; et al. A single-cell RNA expression atlas of normal, preneoplastic and tumorigenic states in the human breast. EMBO J. 2021, 40, e107333.
- Hu, Q.; Hong, Y.; Qi, P.; Lu, G.; Mai, X.; Xu, S.; He, X.; Guo, Y.; Gao, L.; Jing, Z.; et al. Atlas of breast cancer infiltrated B-lymphocytes revealed by paired single-cell RNA-sequencing and antigen receptor profiling. Nat. Commun. 2021, 12, 2186.
- Miligy, I.; Mohan, P.; Gaber, A.; Aleskandarany, M.A.; Nolan, C.C.; Diez-Rodriguez, M.; Mukherjee, A.; Chapman, C.; Ellis, I.O.; Green, A.R.; et al. Prognostic significance of tumour infiltrating B lymphocytes in breast ductal carcinoma in situ. Histopathology 2017, 71, 258–268.
- deLeeuw, R.J.; Kroeger, D.R.; Kost, S.E.; Chang, P.-P.; Webb, J.R.; Nelson, B.H. CD25 Identifies a Subset of CD4+ FoxP3− TIL That Are Exhausted Yet Prognostically Favorable in Human Ovarian Cancer. Cancer Immunol. Res. 2015, 3, 245–253.
- Yeong, J.; Lim, J.C.T.; Lee, B.; Li, H.; Chia, N.; Ong, C.C.H.; Lye, W.K.; Putti, T.C.; Dent, R.; Lim, E.; et al. High Densities of Tumor-Associated Plasma Cells Predict Improved Prognosis in Triple Negative Breast Cancer. Front. Immunol. 2018, 9, 1209.
- Montfort, A.; Pearce, O.; Maniati, E.; Vincent, B.G.; Bixby, L.; Böhm, S.; Dowe, T.; Wilkes, E.H.; Chakravarty, P.; Thompson, R.; et al. A Strong B-cell Response Is Part of the Immune Landscape in Human High-Grade Serous Ovarian Metastases. Clin. Cancer Res. 2017, 23, 250–262.
- Wieczorek, M.; Braicu, E.I.; Oliveira-Ferrer, L.; Sehouli, J.; Blanchard, V. Immunoglobulin G Subclass-Specific Glycosylation Changes in Primary Epithelial Ovarian Cancer. Front. Immunol. 2020, 11, 654.
- Gerçel-Taylor, C.; Bazzett, L.B.; Taylor, D.D. Presence of aberrant tumor-reactive immunoglobulins in the circulation of patients with ovarian cancer. Gynecol. Oncol. 2001, 81, 71–76.
- Ruhaak, L.R.; Kim, K.; Stroble, C.; Taylor, S.L.; Hong, Q.; Miyamoto, S.; Lebrilla, C.B.; Leiserowitz, G. Protein-Specific Differential Glycosylation of Immunoglobulins in Serum of Ovarian Cancer Patients. J. Proteome Res. 2016, 15, 1002–1010.
- Qian, Y.; Wang, Y.; Zhang, X.; Zhou, L.; Zhang, Z.; Xu, J.; Ruan, Y.; Ren, S.; Xu, C.; Gu, J. Quantitative Analysis of Serum IgG Galactosylation Assists Differential Diagnosis of Ovarian Cancer. J. Proteome Res. 2013, 12, 4046–4055.
- Alley, W.R.; Vasseur, J.A.; Goetz, J.A.; Svoboda, M.; Mann, B.F.; Matei, D.E.; Menning, N.; Hussein, A.; Mechref, Y.; Novotny, M.V. N-linked Glycan Structures and Their Expressions Change in the Blood Sera of Ovarian Cancer Patients. J. Proteome Res. 2012, 11, 2282–2300.
- Saldova, R.; Royle, L.; Radcliffe, C.M.; Abd Hamid, U.M.; Evans, R.; Arnold, J.N.; Banks, R.E.; Hutson, R.; Harvey, D.J.; Antrobus, R.; et al. Ovarian Cancer is Associated with Changes in Glycosylation in Both Acute-Phase Proteins and IgG. Glycobiology 2007, 17, 1344–1356.
- Gaffey, M.J.; Frierson, H.F.; Mills, S.E.; Boyd, J.C.; Zarbo, R.J.; Simpson, J.F.; Gross, L.K.; Weiss, L.M. Medullary carcinoma of the breast. Identification of lymphocyte subpopulations and their significance. Mod. Pathol. 1993, 6, 721–728.
- Ito, T.; Saga, S.; Nagayoshi, S.; Imai, M.; Aoyama, A.; Yokoi, T.; Hoshino, M. Class distribution of immunoglobulin-containing plasma cells in the stroma of medullary carcinoma of breast. Breast Cancer Res. Treat. 1986, 7, 97–103.
- Biswas, S.; Mandal, G.; Payne, K.K.; Anadon, C.M.; Gatenbee, C.D.; Chaurio, R.A.; Costich, T.L.; Moran, C.; Harro, C.M.; Rigolizzo, K.E.; et al. IgA transcytosis and antigen recognition govern ovarian cancer immunity. Nature 2021, 591, 464–470.
- Sahin, U.; Türeci, O.; Schmitt, H.; Cochlovius, B.; Johannes, T.; Schmits, R.; Stenner, F.; Luo, G.; Schobert, I.; Pfreundschuh, M. Human neoplasms elicit multiple specific immune responses in the autologous host. Proc. Natl. Acad. Sci. USA 1995, 92, 11810–11813.
- Fosså, A.; Alsøe, L.; Crameri, R.; Funderud, S.; Gaudernack, G.; Smeland, E.B. Serological cloning of cancer/testis antigens expressed in prostate cancer using cDNA phage surface display. Cancer Immunol. Immunother. CII 2004, 53, 431–438.
- Chen, Y.-T.; Scanlan, M.J.; Sahin, U.; Türeci, Ö.; Gure, A.O.; Tsang, S.; Williamson, B.; Stockert, E.; Pfreundschuh, M.; Old, L.J. A testicular antigen aberrantly expressed in human cancers detected by autologous antibody screening. Proc. Natl. Acad. Sci. USA 1997, 94, 1914–1918.
- Gnjatic, S.; Ritter, E.; Büchler, M.W.; Giese, N.A.; Brors, B.; Frei, C.; Murray, A.; Halama, N.; Zörnig, I.; Chen, Y.-T.; et al. Seromic profiling of ovarian and pancreatic cancer. Proc. Natl. Acad. Sci. USA 2010, 107, 5088–5093.
- Blixt, O.; Bueti, D.; Burford, B.; Allen, D.; Julien, S.; Hollingsworth, M.; Gammerman, A.; Fentiman, I.; Taylor-Papadimitriou, J.; Burchell, J.M. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011, 13, R25.
- Richards, E.R.; Devine, P.L.; Quin, R.J.; Fontenot, J.D.; Ward, B.G.; McGuckin, M.A. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol. Immunother. 1998, 46, 245–252.
- Kulić, A.; Sirotković-Skerlev, M.; Jelisavac-Cosić, S.; Herceg, D.; Kovac, Z.; Vrbanec, D. Anti-p53 antibodies in serum: Relationship to tumor biology and prognosis of breast cancer patients. Med. Oncol. 2010, 27, 887–893.
- Lenner, P.; Wiklund, F.; Emdin, S.O.; Arnerlöv, C.; Eklund, C.; Hallmans, G.; Zentgraf, H.; Dillner, J. Serum antibodies against p53 in relation to cancer risk and prognosis in breast cancer: A population-based epidemiological study. Br. J. Cancer 1999, 79, 927–932.
- Erić-Nikolić, A.; Milovanović, Z.; Sánchez, D.; Pekáriková, A.; Džodić, R.; Matić, I.Z.; Tučková, L.; Jevrić, M.; Buta, M.; Rašković, S.; et al. Overexpression of calreticulin in malignant and benign breast tumors: Relationship with humoral immunity. Oncology 2012, 82, 48–55.
- Desmetz, C.; Bascoul-Mollevi, C.; Rochaix, P.; Lamy, P.-J.; Kramar, A.; Rouanet, P.; Maudelonde, T.; Mangé, A.; Solassol, J. Identification of a New Panel of Serum Autoantibodies Associated with the Presence of In situ Carcinoma of the Breast in Younger Women. Clin. Cancer Res. 2009, 15, 4733–4741.
- Honnorat, J.; Cartalat-Carel, S.; Ricard, D.; Camdessanche, J.P.; Carpentier, A.F.; Rogemond, V.; Chapuis, F.; Aguera, M.; Decullier, E.; Duchemin, A.M.; et al. Onco-neural antibodies and tumour type determine survival and neurological symptoms in paraneoplastic neurological syndromes with Hu or CV2/CRMP5 antibodies. J. Neurol. Neurosurg. Psychiatry 2009, 80, 412–416.
- Eichler, T.W.; Totland, C.; Haugen, M.; Qvale, T.H.; Mazengia, K.; Storstein, A.; Haukanes, B.I.; Vedeler, C.A. CDR2L Antibodies: A New Player in Paraneoplastic Cerebellar Degeneration. PLoS ONE 2013, 8, e66002.
- Jarius, S.; Wildemann, B. “Medusa head ataxia”: The expanding spectrum of Purkinje cell antibodies in autoimmune cerebellar ataxia. Part 2: Anti-PKC-gamma, anti-GluR-delta2, anti-Ca/ARHGAP26 and anti-VGCC. J. Neuroinflamm. 2015, 12, 167.
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844.
- Garaud, S.; Zayakin, P.; Buisseret, L.; Rulle, U.; Silina, K.; de Wind, A.; Van den Eyden, G.; Larsimont, D.; Willard-Gallo, K.; Linē, A. Antigen Specificity and Clinical Significance of IgG and IgA Autoantibodies Produced in situ by Tumor-Infiltrating B Cells in Breast Cancer. Front. Immunol. 2018, 9, 2660.
- Meylan, M.; Petitprez, F.; Becht, E.; Bougoüin, A.; Pupier, G.; Calvez, A.; Giglioli, I.; Verkarre, V.; Lacroix, G.; Verneau, J.; et al. Tertiary lymphoid structures generate and propagate anti-tumor antibody-producing plasma cells in renal cell cancer. Immunity 2022, 55, 527–541.e5.
- Coronella, J.A.; Spier, C.; Welch, M.; Trevor, K.T.; Stopeck, A.T.; Villar, H.; Hersh, E.M. Antigen-driven oligoclonal expansion of tumor-infiltrating B cells in infiltrating ductal carcinoma of the breast. J. Immunol. 2002, 169, 1829–1836.
- Odendahl, M.; Mei, H.; Hoyer, B.F.; Jacobi, A.M.; Hansen, A.; Muehlinghaus, G.; Berek, C.; Hiepe, F.; Manz, R.; Radbruch, A.; et al. Generation of migratory antigen-specific plasma blasts and mobilization of resident plasma cells in a secondary immune response. Blood 2005, 105, 1614–1621.
- Martinet, L.; Garrido, I.; Filleron, T.; Le Guellec, S.; Bellard, E.; Fournie, J.-J.; Rochaix, P.; Girard, J.-P. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 2011, 71, 5678–5687.
- Truxova, I.; Kasikova, L.; Hensler, M.; Skapa, P.; Laco, J.; Pecen, L.; Belicova, L.; Praznovec, I.; Halaska, M.J.; Brtnicky, T.; et al. Mature dendritic cells correlate with favorable immune infiltrate and improved prognosis in ovarian carcinoma patients. J. Immunother. Cancer 2018, 6, 139.
- Ukita, M.; Hamanishi, J.; Yoshitomi, H.; Yamanoi, K.; Takamatsu, S.; Ueda, A.; Suzuki, H.; Hosoe, Y.; Furutake, Y.; Taki, M.; et al. Tertiary lymphoid structures induced by CXCL13-producing CD4+ T cells increase tumor infiltrating CD8+ T cells and B cells in ovarian cancer. bioRxiv 2021.
- Sautès-Fridman, C.; Verneau, J.; Sun, C.-M.; Moreira, M.; Chen, T.W.-W.; Meylan, M.; Petitprez, F.; Fridman, W.H. Tertiary Lymphoid Structures and B cells: Clinical impact and therapeutic modulation in cancer. Semin. Immunol. 2020, 48, 101406.
- Griss, J.; Bauer, W.; Wagner, C.; Simon, M.; Chen, M.; Grabmeier-Pfistershammer, K.; Maurer-Granofszky, M.; Roka, F.; Penz, T.; Bock, C.; et al. B cells sustain inflammation and predict response to immune checkpoint blockade in human melanoma. Nat. Commun. 2019, 10, 4186.
- Wieland, A.; Patel, M.R.; Cardenas, M.A.; Eberhardt, C.S.; Hudson, W.H.; Obeng, R.C.; Griffith, C.C.; Wang, X.; Chen, Z.G.; Kissick, H.T.; et al. Defining HPV-specific B cell responses in patients with head and neck cancer. Nature 2021, 597, 274–278.
- Nzula, S.; Going, J.J.; Stott, D.I. Antigen-driven clonal proliferation, somatic hypermutation, and selection of B lymphocytes infiltrating human ductal breast carcinomas. Cancer Res. 2003, 63, 3275–3280.
- Mazor, R.D.; Nathan, N.; Gilboa, A.; Stoler-Barak, L.; Moss, L.; Solomonov, I.; Hanuna, A.; Divinsky, Y.; Shmueli, M.D.; Hezroni, H.; et al. Tumor-reactive antibodies evolve from non-binding and autoreactive precursors. Cell 2022, 185, 1208–1222.e21.
- Small, M.; Treilleux, I.; Couillault, C.; Pissaloux, D.; Picard, G.; Paindavoine, S.; Attignon, V.; Wang, Q.; Rogemond, V.; Lay, S.; et al. Genetic alterations and tumor immune attack in Yo paraneoplastic cerebellar degeneration. Acta Neuropathol. 2018, 135, 569–579.
- Bosisio, F.M.; Wilmott, J.S.; Volders, N.; Mercier, M.; Wouters, J.; Stas, M.; Blokx, W.A.; Massi, D.; Thompson, J.F.; Scolyer, R.A.; et al. Plasma cells in primary melanoma. Prognostic significance and possible role of IgA. Mod. Pathol. 2016, 29, 347–358.
- Cipponi, A.; Mercier, M.; Seremet, T.; Baurain, J.-F.; Théate, I.; van den Oord, J.; Stas, M.; Boon, T.; Coulie, P.G.; van Baren, N. Neogenesis of lymphoid structures and antibody responses occur in human melanoma metastases. Cancer Res. 2012, 72, 3997–4007.
- Aversa, G.; Cocks, B.G.; Punnonen, J.; Carballido, J.M.; de Vries, J.E. Contact-mediated signals and cytokines involved in B-cell activation and isotype switching in pre-B and mature B cells. Res. Immunol. 1994, 145, 222–226, discussion 244–249.
- Coffman, R.L.; Lebman, D.A.; Rothman, P. Mechanism and regulation of immunoglobulin isotype switching. Adv. Immunol. 1993, 54, 229–270.
- Morita, R.; Schmitt, N.; Bentebibel, S.-E.; Ranganathan, R.; Bourdery, L.; Zurawski, G.; Foucat, E.; Dullaers, M.; Oh, S.; Sabzghabaei, N.; et al. Human blood CXCR5(+)CD4(+) T cells are counterparts of T follicular cells and contain specific subsets that differentially support antibody secretion. Immunity 2011, 34, 108–121.
- Noël, G.; Fontsa, M.L.; Garaud, S.; De Silva, P.; de Wind, A.; Van den Eynden, G.G.; Salgado, R.; Boisson, A.; Locy, H.; Thomas, N.; et al. Functional Th1-oriented T follicular helper cells that infiltrate human breast cancer promote effective adaptive immunity. J. Clin. Investig. 2021, 131, e139905.
- Karagiannis, P.; Gilbert, A.E.; Josephs, D.H.; Ali, N.; Dodev, T.; Saul, L.; Correa, I.; Roberts, L.; Beddowes, E.; Koers, A.; et al. IgG4 subclass antibodies impair antitumor immunity in melanoma. J. Clin. Investig. 2013, 123, 1457–1474.
- Mosmann, T.R.; Sad, S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol. Today 1996, 17, 138–146.
- Kurte, M.; López, M.; Aguirre, A.; Escobar, A.; Aguillón, J.C.; Charo, J.; Larsen, C.G.; Kiessling, R.; Salazar-Onfray, F. A synthetic peptide homologous to functional domain of human IL-10 down-regulates expression of MHC class I and Transporter associated with Antigen Processing 1/2 in human melanoma cells. J. Immunol. 2004, 173, 1731–1737.
- Wei, H.; Fu, P.; Yao, M.; Chen, Y.; Du, L. Breast cancer stem cells phenotype and plasma cell-predominant breast cancer independently indicate poor survival. Pathol. Res. Pract. 2016, 212, 294–301.
- Yang, Z.; Wang, W.; Zhao, L.; Wang, X.; Gimple, R.C.; Xu, L.; Wang, Y.; Rich, J.N.; Zhou, S. Plasma cells shape the mesenchymal identity of ovarian cancers through transfer of exosome-derived microRNAs. Sci. Adv. 2021, 7, eabb0737.
- Saqi, A.; Yun, S.S.; Yu, G.H.; Alexis, D.; Taub, R.N.; Powell, C.A.; Borczuk, A.C. Utility of CD138 (syndecan-1) in distinguishing carcinomas from mesotheliomas. Diagn. Cytopathol. 2005, 33, 65–70.
- Lohr, M.; Edlund, K.; Botling, J.; Hammad, S.; Hellwig, B.; Othman, A.; Berglund, A.; Lambe, M.; Holmberg, L.; Ekman, S.; et al. The prognostic relevance of tumour-infiltrating plasma cells and immunoglobulin kappa C indicates an important role of the humoral immune response in non-small cell lung cancer. Cancer Lett. 2013, 333, 222–228.
- Schmidt, M.; Hellwig, B.; Hammad, S.; Othman, A.; Lohr, M.; Chen, Z.; Boehm, D.; Gebhard, S.; Petry, I.; Lebrecht, A.; et al. A Comprehensive Analysis of Human Gene Expression Profiles Identifies Stromal Immunoglobulin κ C as a Compatible Prognostic Marker in Human Solid Tumors. Clin. Cancer Res. 2012, 18, 2695–2703.
- McCarron, M.J.; Park, P.W.; Fooksman, D.R. CD138 mediates selection of mature plasma cells by regulating their survival. Blood 2017, 129, 2749–2759.
- Iglesia, M.D.; Vincent, B.G.; Parker, J.S.; Hoadley, K.; Carey, L.A.; Perou, C.M.; Serody, J.S. Prognostic B-Cell Signatures using mRNA-Seq in Patients with Subtype-Specific Breast and Ovarian Cancer. Clin. Cancer Res. 2014, 20, 3818–3829.
- Gentles, A.J.; Newman, A.M.; Liu, C.L.; Bratman, S.V.; Feng, W.; Kim, D.; Nair, V.S.; Xu, Y.; Khuong, A.; Hoang, C.D.; et al. The prognostic landscape of genes and infiltrating immune cells across human cancers. Nat. Med. 2015, 21, 938–945.
- Ridolfi, R.L.; Rosen, P.P.; Port, A.; Kinne, D.; Miké, V. Medullary carcinoma of the breast. A clinicopathologic study with 10 year follow-up. Cancer 1977, 40, 1365–1385.
- Kuroda, H.; Jamiyan, T.; Yamaguchi, R.; Kakumoto, A.; Abe, A.; Harada, O.; Enkhbat, B.; Masunaga, A. Prognostic value of tumor-infiltrating B lymphocytes and plasma cells in triple-negative breast cancer. Breast Cancer 2021, 28, 904–914.
- Fan, C.; Prat, A.; Parker, J.S.; Liu, Y.; Carey, L.A.; Troester, M.A.; Perou, C.M. Building prognostic models for breast cancer patients using clinical variables and hundreds of gene expression signatures. BMC Med. Genom. 2011, 4, 3.
- Harris, R.J.; Cheung, A.; Ng, J.C.F.; Laddach, R.; Chenoweth, A.M.; Crescioli, S.; Fittall, M.; Dominguez-Rodriguez, D.; Roberts, J.; Levi, D.; et al. Tumor-Infiltrating B Lymphocyte Profiling Identifies IgG-Biased, Clonally Expanded Prognostic Phenotypes In Triple-Negative Breast Cancer. Cancer Res. 2021, 81, 4290–4304.
- Bolotin, D.A.; Poslavsky, S.; Davydov, A.N.; Frenkel, F.E.; Fanchi, L.; Zolotareva, O.I.; Hemmers, S.; Putintseva, E.V.; Obraztsova, A.S.; Shugay, M.; et al. Antigen receptor repertoire profiling from RNA-seq data. Nat. Biotechnol. 2017, 35, 908–911.
- Isaeva, O.I.; Sharonov, G.V.; Serebrovskaya, E.O.; Turchaninova, M.A.; Zaretsky, A.R.; Shugay, M.; Chudakov, D.M. Intratumoral immunoglobulin isotypes predict survival in lung adenocarcinoma subtypes. J. Immunother. Cancer 2019, 7, 279.
- Welinder, C.; Jirström, K.; Lehn, S.; Nodin, B.; Marko-Varga, G.; Blixt, O.; Danielsson, L.; Jansson, B. Intra-tumour IgA1 is common in cancer and is correlated with poor prognosis in bladder cancer. Heliyon 2016, 2, e00143.
- Wang, H.; Chen, D.; Wang, R.; Quan, W.; Xia, D.; Mei, J.; Xu, J.; Liu, C. NY-ESO-1 expression in solid tumors predicts prognosis. Medicine 2019, 98, e17990.
- Patil, N.S.; Nabet, B.Y.; Müller, S.; Koeppen, H.; Zou, W.; Giltnane, J.; Au-Yeung, A.; Srivats, S.; Cheng, J.H.; Takahashi, C.; et al. Intratumoral plasma cells predict outcomes to PD-L1 blockade in non-small cell lung cancer. Cancer Cell 2022, 40, 289–300.e4.
- Hollern, D.P.; Xu, N.; Thennavan, A.; Glodowski, C.; Garcia-Recio, S.; Mott, K.R.; He, X.; Garay, J.P.; Carey-Ewend, K.; Marron, D.; et al. B Cells and T Follicular Helper Cells Mediate Response to Checkpoint Inhibitors in High Mutation Burden Mouse Models of Breast Cancer. Cell 2019, 179, 1191–1206.e21.
- Sánchez-Alonso, S.; Setti-Jerez, G.; Arroyo, M.; Hernández, T.; Martos, M.I.; Sánchez-Torres, J.M.; Colomer, R.; Ramiro, A.R.; Alfranca, A. A new role for circulating T follicular helper cells in humoral response to anti-PD-1 therapy. J. Immunother. Cancer 2020, 8, e001187.
This entry is offline, you can click here to edit this entry!
 Encyclopedia
Encyclopedia
