Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The thiazolidin-2,4-dione (TZD) moiety plays a central role in the biological functioning of several essential molecules. The availability of substitutions at the third and fifth positions of the Thiazolidin-2,4-dione (TZD) scaffold makes it a highly utilized and versatile moiety that exhibits a wide range of biological activities.
- thiazolidin-2,4-dione derivatives
- antimicrobial activity
- antioxidant activity
1. Introduction
Small heterocyclic structures containing nitrogen and sulfur have been under investigation for a long time owing to their therapeutic relevance. They offer a wide range of structural varieties and also possess a proven range of diversified therapeutic potentials. Amongst the extensive variety of heterocyclic scaffolds explored in the search for potent bioactive molecules in the process of drug discovery, the thiazolidin-2,4-dione (TZD) (Figure 1) ring system has been acknowledged as a significant scaffold in medicinal chemistry [1].
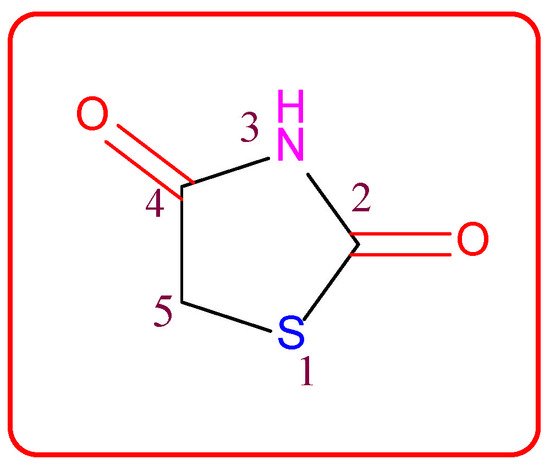
Figure 1. Structure of the thiazolidine-2,4-dione scaffold.
Thiazolidin-2,4-dione (Figure 1), also called glitazone, is a heterocyclic moiety that consists of a five-membered saturated thiazolidine ring with sulfur at 1 and nitrogen at 3, along with two carbonyl functional groups at the 2 and 4 positions. Substitutions of various moieties are possible only at the third and fifth positions of the Thiazolidin-2,4-dione (TZD) scaffold. The analogues of TZD offer a wide range of structural varieties [2] and also possess a proven range of diversified therapeutic potentials, such as antidiabetic [3][4][5], analgesic, anti-inflammatory [6][7][8], wound healing [9], antiproliferative [10][11][12][13][14], antimalarial [15], antitubercular [16][17], hypolipidemic [18], antiviral [19], antimicrobial, antifungal [20][21][22][23], and antioxidant properties [24][25], etc.
2. The History of Glitazones as Antidiabetics
TZDs are primarily used as hypoglycemic agents over a long time. Ciglitazone is the prototype of the TZD class, which was developed by Takeda Pharmaceuticals (Japan) in the early 1980s but has never been used as a medication due to its hepatotoxicity. In the year 1988, another TZD analog, named troglitazone, was developed by the Sankyo Company (Japan) as an antidiabetic agent, but it was also banned in the year 2000 due to its hepatic toxicity. In 1999, Pfizer (USA) and Takeda (Japan) jointly developed two molecules, pioglitazone (patented in 2002), and englitazone, and subsequently, Pfizer and Smithkline also developed rosiglitazone and darglitazone in the same year. Englitazone and darglitazone were discontinued due to their hepatotoxicity. However, pioglitazone was reported to be safe for use in the hepatic system and is currently in use as an antidiabetic agent. In 2001, rosiglitazone was reported to cause heart failure due to fluid retention and, hence, the Food and Drug Administration (FDA) restricted its use in the year 2010. However, in the year 2013, the restriction was removed by the FDA after a series of trials proved that there was no effect on the heart due to fluid retention. Another drug, lobeglitazone, was developed by Chong Kun Dang Pharmaceuticals (Korea) in 2013, which was approved by the Ministry of Food and Drug Safety of Korea. Some other molecules, such as netaglitazone, rivoglitazone, and balaglitazone, were also developed but then withdrawn during the clinical trial due to their severe toxicities, and these never came to the market. The structures of various TZDs are shown in Figure 2 [26][27].
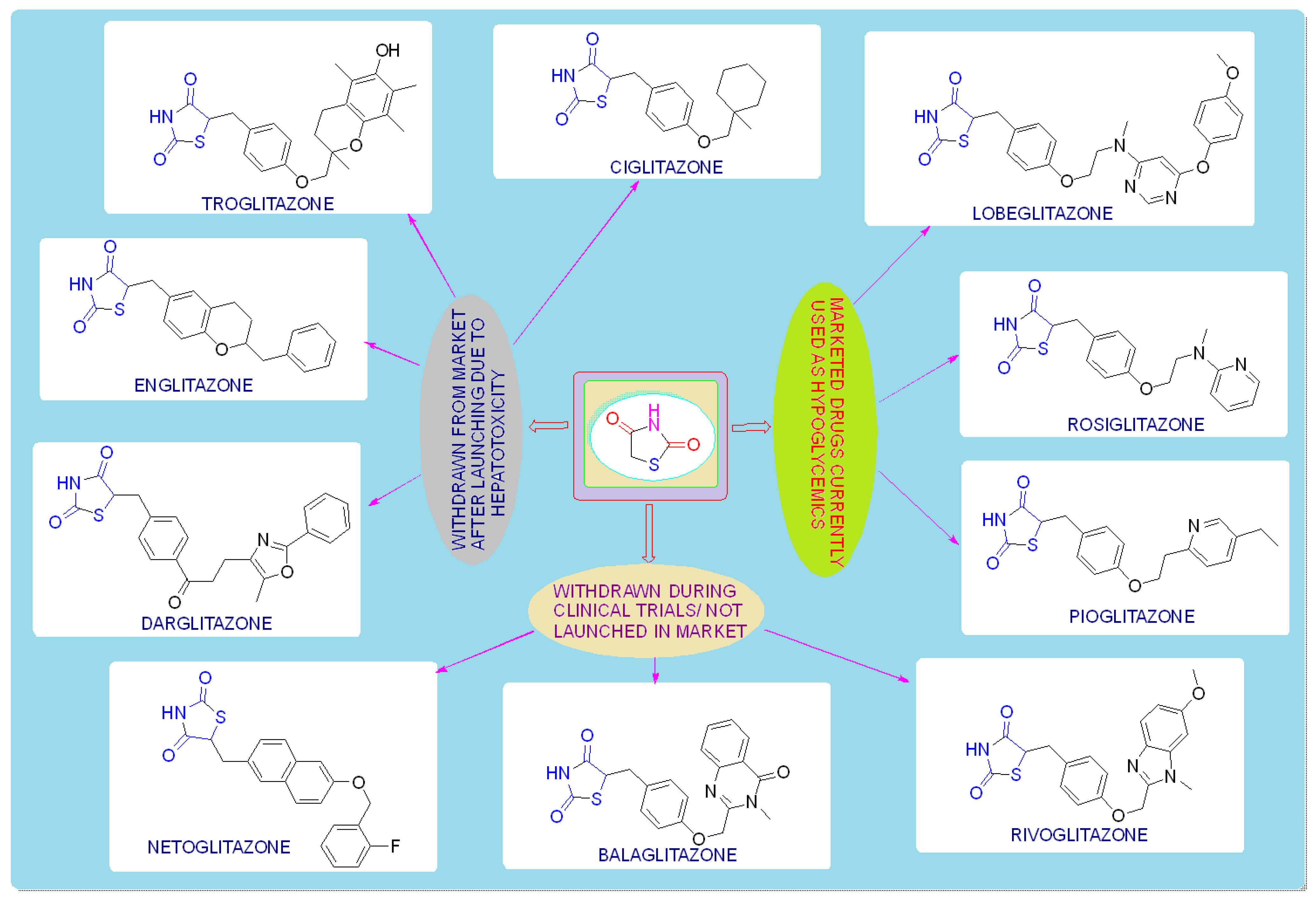
Figure 2. Antidiabetic molecules developed with the thiazolidin-2,4-dione moiety.
3. Mechanism of Action
TZDs produce their biological response by stimulating the PPARγ receptor (antidiabetic activity) and cytoplasmic Mur ligase enzyme (antimicrobial activity), and by scavenging ROS (antioxidant activity).
-
Family of PPARs:
PPARs are a subfamily of transducer proteins that act as transcriptional factors, belonging to the nuclear superfamily of retinoic acid receptors (RARs)/steroid receptors/thyroid hormone receptors (TRs), which are involved in different processes and also help in the regulation and expression of various genes that are vital for glucose and lipid metabolism [28][29]. These nuclear receptors were first identified in rodents in the year 1990s [26].
-
Isoforms of PPARs:
To date, three different types of PPARs have been identified, i.e., PPAR-α (NR1C1), PPAR-β/δ (NR1C2), and PPAR-γ (NR1C3), which are encoded by different genes.
-
Structure and biological functions of PPARs:
All of the PPAR isoforms have similar functional and structural features. The structure of PPAR has four principal functional domains, known as A/B, C, D, and E/F (Figure 3). The domain A/B, located at the NH2-terminal, comprises ligand-independent activation function 1 (AF-1) and causes the phosphorylation of PPAR. The domain C, also called the DNA-binding domain (DBD), has two zinc atoms that promote the binding of the peroxisome proliferator response element (PPRE) to PPAR in the target gene promoter region. The D site functions as a docking domain for the binding of the coactivators/corepressors to the DNA receptors. The E/F domain near the AF-2 region, also called the ligand-binding domain (LBD), is used to specify the ligand and also activates the binding of PPAR to PPRE, thereby promoting the targeted gene expression. The ligand-dependent activation function 2 (AF-2) recruits the PPAR co-factors that assist in the gene transcription processes. Their biological functions and distribution in the tissues, along with their agonists [26][30][31][32].

Figure 3. Principal functional domains of PPARs.
3.1. Mechanism of the TZD as an Antidiabetic
PPAR acts by either transactivation or by trans-repression to enact its antidiabetic activity. In transactivation, PPAR is activated upon binding with the exogenous ligand (TZD) or endogenous ligands (PGs, fatty acids, etc.). PPAR then heterodimerizes with the retinoid X factor (RXR) to form the PPAR–RXR complex. This complex binds with peroxisome proliferator response elements (PPRE) in the target gene with a coactivator that has histone acetylase activity and promotes the transcription of different genes involved in the cellular differentiation and glucose and lipid metabolism (Figure 4) [26][28]. In trans-repression, PPARs interact negatively with other signal transduction pathways, such as the nuclear factor kappa beta (NFκB) pathway, which controls various genes involved in inflammation and also regulates inflammatory mediators, such as leukocytes and cytokines, etc. (Figure 5) [28].
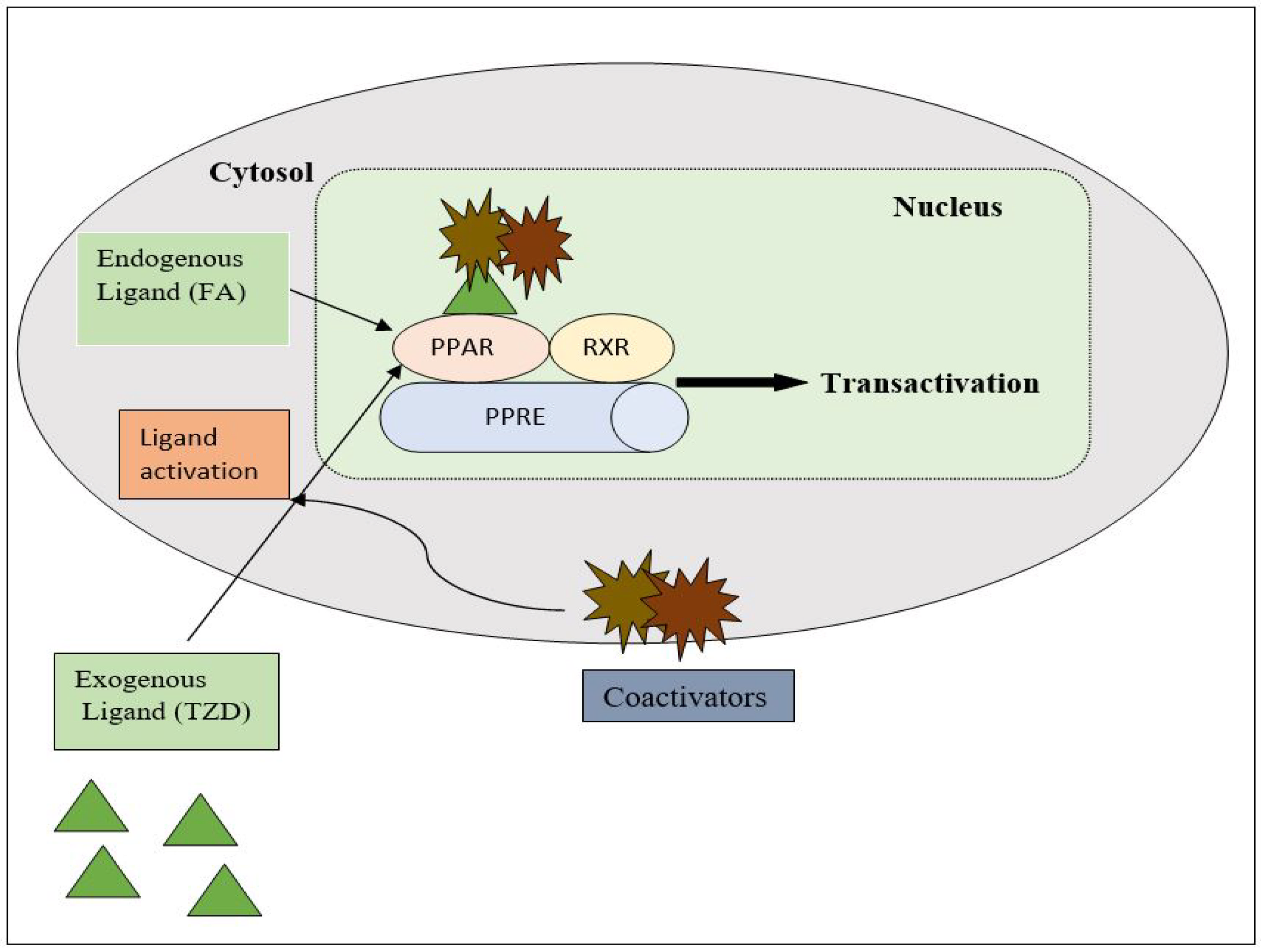
Figure 4. Gene transcription mechanisms of PPAR.
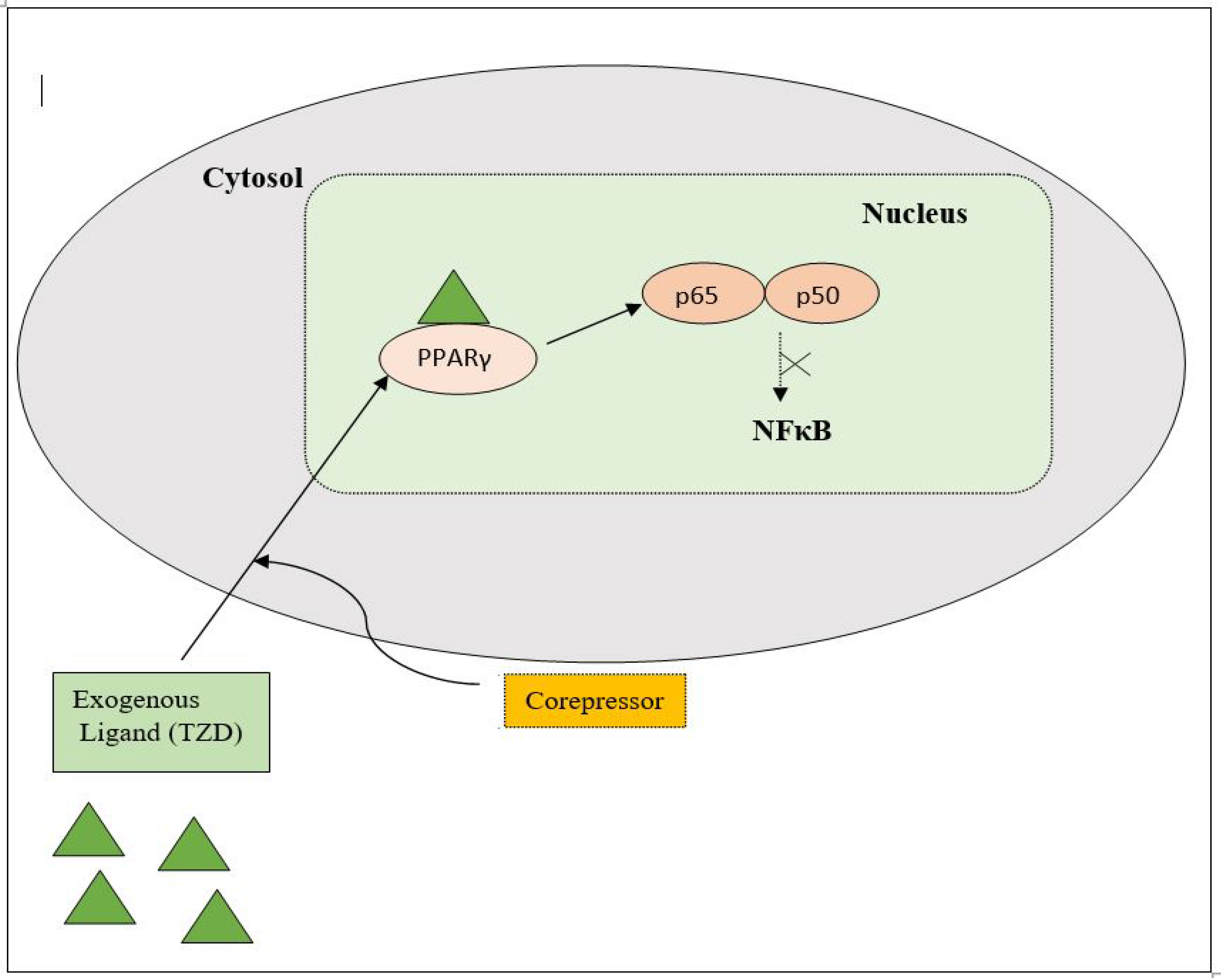
Figure 5. Gene trans-repression mechanisms of PPAR.
Thiazolidinediones (TZDs) are the most important synthetic moieties with PPAR-γ activation properties, which improve insulin resistance and, hence, lower blood glucose levels in type-II diabetes. In adipose tissues, TZDs activate PPAR-γ, which causes lipid uptake and the storage of triglycerides (TGs). White adipose tissues (WAT) then take up free fatty acids (FFAs) from the tissues (skeletal muscle, liver), where their growth obstructs insulin signaling, known as the lipid steal hypothesis. PPAR-γ also mediates the production of adipocytes. In macrophages, TZDs directly activate PPAR-γ, causing an anti-inflammatory M2 phenotype which leads to a decreased macrophage infiltration in the WAT. TZDs also help to reduce fibrosis and inflammation by acting on PPAR-γ in Kupffer and stellate cells, as well as the parenchymal cells of the steatosis liver, and also mediate atherosclerosis by interacting with PPAR-γ in the macrophages (Figure 6) [26][32][33].
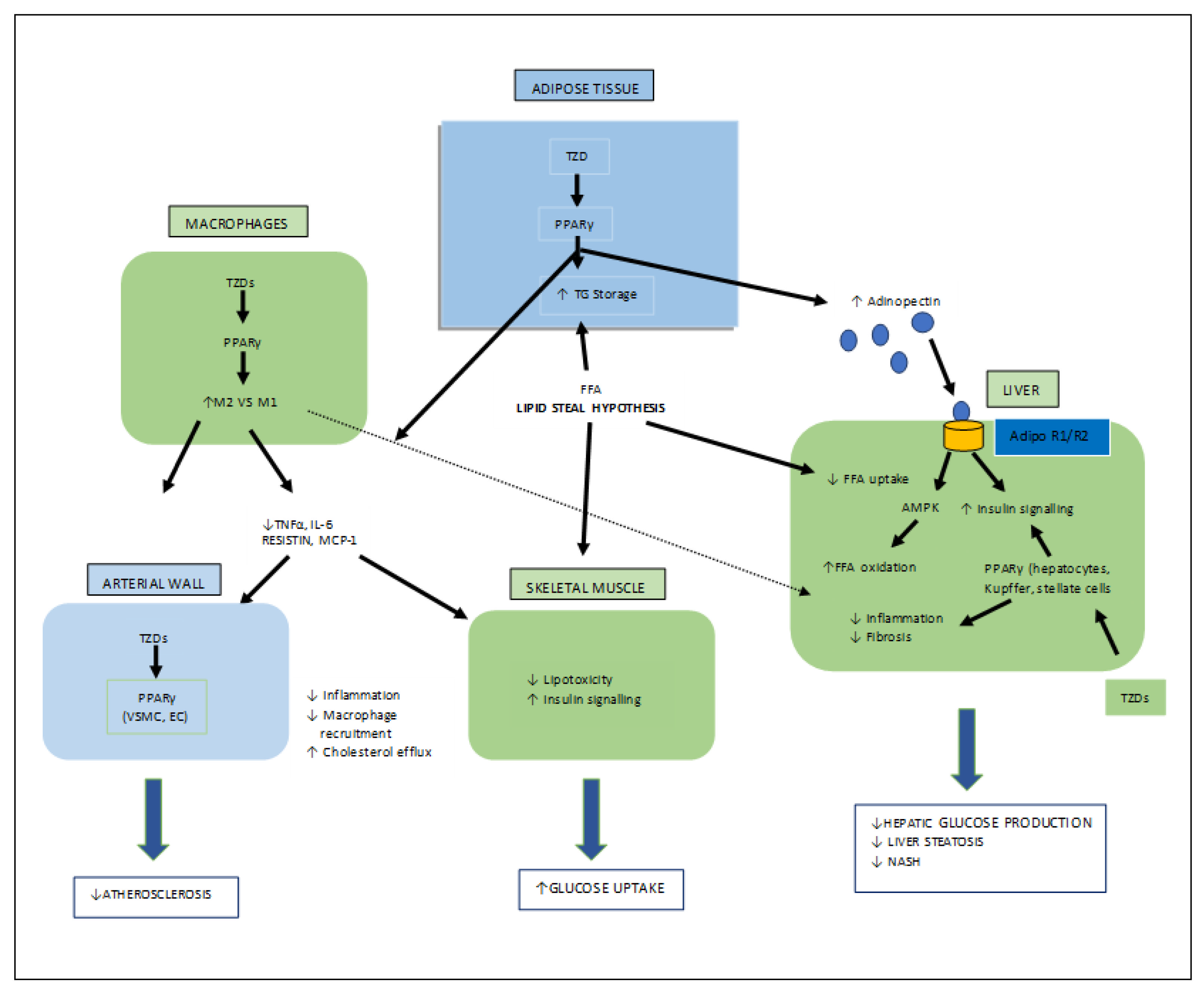
Figure 6. Various target organs/sites of TZD-PPARγ.
3.2. Mechanism of TZD as Antimicrobial Agent
For bacterial viability, the bacterial cell wall is an important component in maintaining the cell shape and protection. Peptidoglycan is one of the major constituents of the bacterial cell wall and is found on the outer wall of cytoplasmic membrane. Its biosynthetic enzyme inhibition can lead to cell death. The enzymes involved in the synthesis can either be membrane-bound extracellular enzymes (penicillin-binding proteins) or cytoplasmic enzymes (Mur enzymes). Peptidoglycan peptide stem biosynthesis involves four ATP-dependent enzymes, known as the Mur ligases (Mur C-F). They mediate the formation of UDP-MurNAc-pentapeptide through the stepwise additions of MurC (L-alanine), MurD (D-glutamic acid), a diamino acid which is generally a meso-diaminopimelic acid, or MurE (L-lysine) and MurF (dipeptide D-Ala-D-Ala) to the D-lactoyl group of UDP-N-acetylmuramic acid (Figure 7). TZD molecules are supposed to inhibit these cytoplasmic ligases and, hence, lead to bacterial cell death [34][35][36][37].
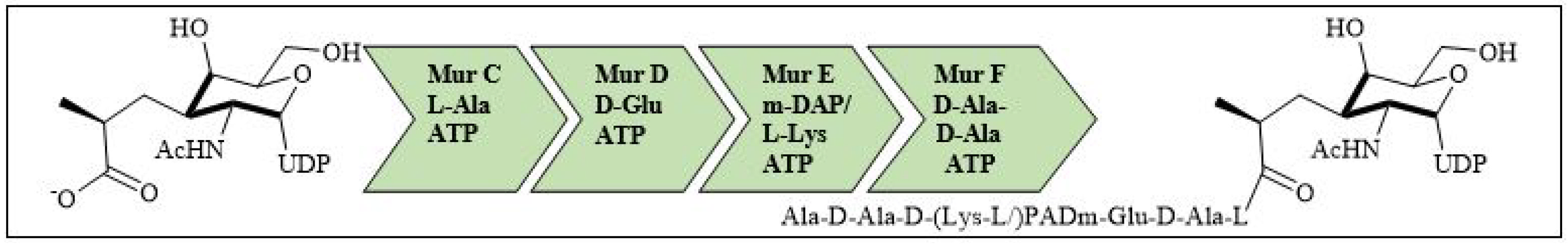
Figure 7. Peptidoglycan peptide stem formation by the Mur ligases enzymes.
3.3. Mechanism of TZD as an Antioxidant
Oxidative stress occurs due to the production of free radicals. Free radicals are chemically active molecules which are either deficient or have a greater number of electrons. Free radicals containing oxygen are the most significant free radicals, also known as reactive oxygen species (ROS). Oxidants activate various relevant enzymes, such as SOD, catalase, and NADPH oxidase, to convert the oxygen to ROS. ROS scavenge body cells in order to capture or donate protons, thus causing cell, proteins, and DNA damage. TZD derivatives are supposed to work by preventing the cascade effect produced through ROS propagation by donating its proton to the ROS (Figure 8) [38][39][40].
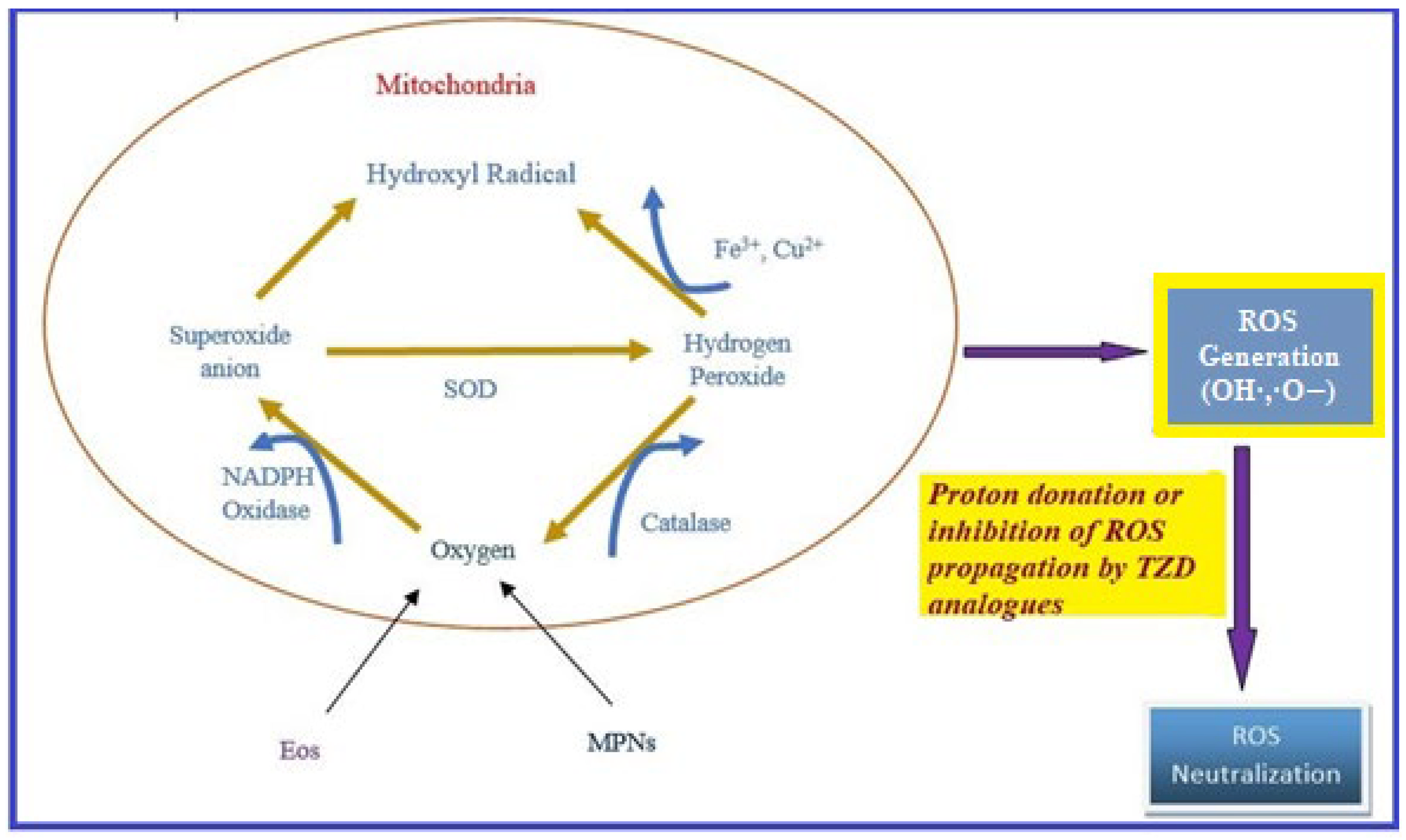
Figure 8. ROS generation and antioxidant scavenging mechanism of TZDs.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196763
References
- Naim, M.J.; Alam, M.J.; Ahmad, S.; Nawaz, F.; Shrivastava, N.; Sahu, M.; Alam, O. Therapeutic journey of 2,4-thiazolidinediones as a versatile scaffold: An insight into structure activity relationship. Eur. J. Med. Chem. 2017, 129, 218–250.
- Kumar, H.; Deep, A.; Marwaha, R.K. Chemical Synthesis, Mechanism of Action and Anticancer Potential of Medicinally Important Thiazolidin-2,4-dione Derivatives: A Review. Mini. Rev. Med. Chem. 2019, 19, 1476–1516.
- Bansal, G.; Singh, S.; Monga, V.; Thanikachalama, P.V.; Chawla, P. Synthesis and biological evaluation of thiazolidine-2,4-dione-pyrazole conjugates as antidiabetic, anti-inflammatory and antioxidant agents. Bioorg. Chem. 2019, 19, 103271.
- Mahapatra, M.K.; Kumar, R.; Kumar, M. Exploring sulfonate esters of 5-arylidene thiazolidine-2,4-diones as PTP1B inhibitors with anti-hyperglycemic activity. Med. Chem. Res. 2018, 27, 476–487.
- Sucheta; Tahlan, S.; Verma, P.K. Synthesis, SAR and in vitro therapeutic potentials of thiazolidine-2,4-diones. Chem. Cent. J. 2018, 12, 129.
- Abdellatif, K.R.; Fadaly, W.A.; Kamel, G.M.; Elshaier, Y.A.; El-Magd, M.A. Design, synthesis, modeling studies and biological evaluation of thiazolidine derivatives containing pyrazole core as potential anti-diabetic PPAR-γ agonists and anti-inflammatory COX-2 selective inhibitors. Bioorg. Chem. 2019, 82, 86–99.
- Elkamhawy, A.; Kim, N.Y.; Hassan, A.H.E.; Park, J.E.; Paik, S.; Yang, J.E.; Oh, K.S.; Lee, B.H.; Lee, M.Y.; Shin, K.J.; et al. Thiazolidine-2,4-dione-based irreversible allosteric IKK-β kinase inhibitors: Optimization into in vivo active anti-inflammatory agents. Eur. J. Med. Chem. 2020, 188, 111955.
- Asati, V.; Bajaj, S.; Mahapatra, D.K.; Bharti, S.K. Molecular Modeling Studies of Some Thiazolidine-2,4-Dione Derivatives as 15-PGDH Inhibitors. Med. Chem. Res. 2015, 25, 94–108.
- Yu, I.; Choi, D.; Lee, H.-K.; Cho, H. Synthesis and Biological Evaluation of New Benzylidenethiazolidine-2,4-Dione Derivatives as 15-Hydroxyprostaglandin Dehydrogenase Inhibitors to Control the Intracellular Levels of Prostaglandin E2 for Wound Healing. Biotechnol. Bioprocess Eng. 2019, 24, 464–475.
- Upadhyay, N.; Tilekar, K.; Jänsch, N.; Schweipert, M.; Hess, J.D.; Henze Macias, L.; Mrowka, P.; Aguilera, R.J.; Choe, J.; Meyer-Almes, F.-J.; et al. Discovery of novel N-substituted thiazolidinediones (TZDs) as HDAC8 inhibitors: In-silico studies, synthesis, and biological evaluation. Bioorg. Chem. 2020, 100, 103934.
- El-Kashef, H.; Badr, G.; Abo El-Maali, N.; Sayed, D.; Melnyk, P.; Lebegue, N.; Abd El-Khalek, R. Synthesis of a novel series of (Z)-3,5-disubstituted thiazolidine-2,4-diones as promising anti-breast cancer agents. Bioorg. Chem. 2020, 96, 103569.
- Abdelgawad, M.A.; El-Adl, K.; El-Hddad, S.S.A.; Elhady, M.M.; Saleh, N.M.; Khalifa, M.M.; Khedr, F.; Alswah, M.; Nayl, A.A.; Ghoneim, M.M.; et al. Design, Molecular Docking, Synthesis, Anticancer and Anti-Hyperglycemic Assessments of Thiazolidine-2,4-diones Bearing Sulfonylthiourea Moieties as Potent VEGFR-2 Inhibitors and PPAR Agonists. Pharmaceuticals 2022, 15, 226.
- Taghour, M.S.; Elkady, H.; Eldehna, W.M.; El-Deeb, N.M.; Kenawy, A.M.; Elkaeed, E.B.; Alsfouk, A.A.; Alesawy, M.S.; Metwaly, A.M.; Eissa, I.H. Design and synthesis of thiazolidine-2,4-diones hybrids with 1,2-dihydroquinolones and 2-oxindoles as potential VEGFR-2 inhibitors: In-vitro anticancer evaluation and in-silico studies. J. Enzym. Inhib. Med. Chem. 2022, 37, 1903–1917.
- El-Adl, K.; Sakr, H.; El-Hddad, S.S.; El-Helby, A.G.; Nasser, M.; Abulkhair, H.S. Design, synthesis, docking, ADMET profile, and anticancer evaluations of novel thiazolidine-2,4-dione derivatives as VEGFR-2 inhibitors. Arch. Pharm. 2021, 354, 2000491.
- Sharma, R.K.; Younis, Y.; Mugumbate, G.; Njoroge, M.; Gut, J.; Rosenthal, P.J.; Chibale, K. Synthesis and structure activity-relationship studies of thiazolidinediones as antiplasmodial inhibitors of the Plasmodium falciparum cysteine protease falcipain-2. Eur. J. Med. Chem. 2015, 90, 507–518.
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Synthesis and antimycobacterial activity of thiazolidine-2,4-dione based derivatives with halogenbenzohydrazones and pyridinecarbohydrazones substituents. Eur. J. Med. Chem. 2020, 189, 112045.
- Trotsko, N.; Golus, J.; Kazimierczak, P.; Paneth, A.; Przekora, A.; Ginalska, G.; Wujec, M. Design, synthesis and antimycobacterial activity of thiazolidine-2,4-dionebased thiosemicarbazone derivatives. Bioorg. Chem. 2020, 97, 103676.
- Mohammed Iqbal, A.K.; Khan, A.Y.; Kalashetti, M.B.; Belavagi, N.S.; Gong, Y.-D.; Khazi, I.A.M. Synthesis, hypoglycemic and hypolipidemic activities of novel thiazolidinedione derivatives containing thiazole/triazole/oxadiazole ring. Eur. J. Med. Chem. 2012, 53, 308–315.
- Bahare, R.S.; Ganguly, S.; Choowongkomon, K.; Seetaha, S. Synthesis, HIV-1 RT inhibitory, antibacterial, antifungal and binding mode studies of some novel N-substituted 5-benzylidine-2, 4-thiazolidinediones. DARU J. Pharm. Sci. 2015, 23, 6.
- Moorthy, P.; Ekambaram, S.P.; Perumal, S.S. Synthesis, characterization and antimicrobial evaluation of imidazolyl thiazolidinedione derivatives. Arab. J. Chem. 2019, 12, 413–419.
- Nastasa, C.M.; Duma, M.; Pîrnău, A.; Vlase, L.; Tiperciuc, B.; Oniga, O. Development of new 5-(chromene-3-yl)methylene-2,4-thiazolidinediones as antimicrobial agents. Clujul Med. 2016, 89, 122–127.
- Mohanty, S.; Reddy, S.G.; RamaDevi, B.; Karmakar, A.C. An assembly of structurally diverse small and simple 5-aminomethylene derivatives of 2,4-thiazolidinedione and studies of their biological activity. Med. Chem. Res. 2015, 24, 4037–4049.
- Levshin, I.B.; Simonov, A.Y.; Lavrenov, S.N.; Panov, A.A.; Grammatikova, N.E.; Alexandrov, A.A.; Ghazy, E.S.; Savin, N.A.; Gorelkin, P.V.; Erofeev, A.S.; et al. Antifungal Thiazolidines: Synthesis and Biological Evaluation of Mycosidine Congeners. Pharmaceuticals 2022, 15, 563.
- Kumar, H.; Deep, A.; Marwaha, R.K. Design, synthesis, in silico studies and biological evaluation of 5-((E)-4-((E)-(substitutedaryl/alkyl)methyl)benzylidene)thiazolidine-2,4-dione derivatives. BMC Chem. 2020, 14, 25.
- Marc, G.; Stana, A.; Oniga, S.D.; Pirnau, A.; Vlase, L.; Oniga, O. New phenolic derivatives of thiazolidine-2,4-dione with antioxidant and antiradical properties: Synthesis, characterization, in vitro evaluation, and quantum studies. Molecules 2019, 24, 2060.
- Bansal, G.; Thanikachalam, P.V.; Maurya, R.K.; Chawla, P.; Ramamurthy, S. An overview on medicinal perspective of thiazolidine-2,4-dione: A remarkable scaffold in the treatment of type 2 diabetes. J. Adv. Res. 2020, 23, 163–205.
- Kim, S.G.; Kim, D.M.; Woo, J.-T.; Jang, H.C.; Chung, C.H.; Ko, K.S.; Park, J.H.; Park, Y.S.; Kim, S.J.; Choi, D.S. Efficacy and Safety of Lobeglitazone Monotherapy in Patients with Type 2 Diabetes Mellitus over 24-Weeks: A Multicenter, Randomized, Double-Blind, Parallel-Group, Placebo Controlled Trial. PLoS ONE 2014, 9, e92843.
- Kota, B.; Huang, T.; Roufogalis, B. An Overview on Biological Mechanisms of PPARs. Pharmacol. Res. 2005, 51, 85–94.
- Yki-Jarvinen, H. Thiazolidinediones. N. Engl. J. Med. 2004, 351, 1106–1118.
- Werman, A.; Hollenberg, A.; Solanes, G.; Bjørbæk, C.; Vidal-Puig, A.J.; Flier, J.S. Ligand-Independent Activation Domain in the N Termsinus of Peroxisome Proliferator-Activated Receptor γ (PPARγ). J. Biol. Chem. 1997, 272, 20230–20235.
- Berger, J.; Moller, D.E. The mechanisms of action of PPARs. Annu. Rev. Med. 2002, 53, 409–435.
- Hauner, H. The mode of action of thiazolidinediones. Diabetes Metab. Res. Rev. 2002, 18, S10–S15.
- Cariou, B.; Charbonnel, B.; Staels, B. Thiazolidinediones and PPARγ agonists: Time for a reassessment. Trends Endocrinol. Metab. 2012, 23, 205–215.
- Jukic, M.; Gobec, S.; Sova, M. Reaching toward underexplored targets in antibacterial drug design. Drug Dev. Res. 2018, 80, 6–10.
- Tomašić, T.; Zidar, N.; Kovač, A.; Turk, S.; Simčič, M.; Blanot, D.; Müller-Premru, M.; Filipič, M.; Grdadolnik, S.G.; Zega, A.; et al. 5-Benzylidenethiazolidin-4-ones as multitarget inhibitors of bacterial Mur ligases. ChemMedChem 2010, 5, 286–295.
- Barreteau, H.; Kovac, A.; Boniface, A.; Sova, M.; Gobec, S.; Blanot, D. Cytoplasmic steps of peptidoglycan biosynthesis. FEMS Microbiol. Rev. 2008, 32, 168–207.
- Sink, R.; Barreteau, H.; Patin, D.; Mengin-Lecreulx, D.; Gobec, S.; Blanot, D. MurD enzymes: Some recent developments. Biomol. Concepts 2013, 4, 539–556.
- Khare, N.; Kapoor, A. Antioxidant evaluation of 2,4-thiazolidinedione and rhodanine derivatives. Der. Pharmacia. Lettre. 2016, 8, 38–46.
- Hossain, S.U.; Bhattacharya, S. Synthesis of O-prenylated and O-geranylated derivatives of 5-benzylidene2,4-thiazolidinediones and evaluation of their free radical scavenging activity as well as effect on some phase II antioxidant/detoxifying enzymes. Bioorg. Med. Chem. Lett. 2007, 17, 1149–1154.
- Jiang, Y.; Wang, X.; Hu, D. Mitochondrial alterations during oxidative stress in chronic obstructive pulmonary disease. Int. J. Chron. Obstruct. Pulmon. Dis. 2017, 12, 1153–1162.
This entry is offline, you can click here to edit this entry!
