Three-finger toxins (abbreviated 3FTx) are a protein superfamily of small toxin proteins found in the venom of snakes. Three-finger toxins are in turn members of a larger superfamily of three-finger protein domains which includes non-toxic proteins that share a similar protein fold. The group is named for its common structure consisting of three beta strand loops connected to a central core containing four conserved disulfide bonds. The 3FP protein domain has no enzymatic activity and is typically between 60-74 amino acid residues long. Despite their conserved structure, three-finger toxin proteins have a wide range of pharmacological effects. Most members of the family are neurotoxins that act on cholinergic intercellular signaling; the alpha-neurotoxin family interacts with muscle nicotinic acetylcholine receptors (nAChRs), the kappa-bungarotoxin family with neuronal nAChRs, and muscarinic toxins with muscarinic acetylcholine receptors (mAChRs).
- acetylcholine receptors
- three-finger toxin
- 3ftx
1. Structure
The three-finger toxin superfamily is defined by a common tertiary structure consisting of three beta strand-containing loops (designated loops I, II, and III) projecting from a small hydrophobic core containing four conserved disulfide bonds. This structure is thought to resemble a hand with three fingers, giving rise to the name.[1] The proteins are typically 60-74 amino acid residues long, though some have additional N- or C-terminal extensions. An additional disulfide bond may be present in either loop I or loop II.[1] The superfamily can be broadly divided into three classes:[1][2][3]
- short-chain toxins have under 66 residues and four core disulfide bonds.
- long-chain toxins have at least 66 residues, a disulfide bond in loop II, and possibly a C-terminal extension.
- non-conventional toxins have a disulfide bond in loop I and possibly terminal extensions.
1.1. Oligomerization
Most 3FTx proteins are monomers. However, some 3FTx subgroups form functional non-covalent homodimers.[1] The kappa-bungarotoxin group is the best characterized dimeric 3FTx, and interacts through an antiparallel dimer interface composed of the outer strand of loop III.[4] Haditoxin is another example of a dimeric 3FTx; it is a member of the short-chain group and has a similar dimer interface but distinct pharmacology compared to the long-chain-like kappa-bungarotoxins.[6]
A few examples of covalently linked dimers have also been described.[1] These proteins, from the non-conventional group, are linked through intermolecular disulfide bonds. Some, such as irditoxin, are heterodimers linked by cysteines in loops I and II.[5] Others, such as alpha-cobrotoxin, can form both homodimers and heterodimers which have distinct pharmacological activities in vitro, though their functional significance is unclear due to their very low concentration in venom.[7]
2. Function
Despite their conserved shared structure, 3FTx proteins have a wide range of pharmacological effects mediating their toxicity. Many members of the family are neurotoxins that bind to receptor proteins in the cell membrane, particularly nicotinic acetylcholine receptors. Others, including the second-largest 3FTx subgroup, are cardiotoxins.[1]
2.1. Cellular Targets
Nicotinic acetylcholine receptors
Many of the most well-characterized 3FTx proteins exert their toxic effects through binding to nicotinic acetylcholine receptors (nAChRs), a family of ligand-gated ion channels. 3FTx binding interferes with cholinergic intercellular signaling particularly at neuromuscular junctions and causes paralysis. The alpha-neurotoxin family is a group of 3FTx proteins that bind muscle nAChRs, preventing the binding of the neurotransmitter acetylcholine[8].[1] Alpha-bungarotoxin, the alpha-neurotoxin from the many-banded krait (Bungarus multicinctus), has a long history of use in molecular biology research; it was through the study of this toxin that nAChRs were isolated and characterized, which facilitated the study of the subunit composition of tissue-specific nAChRs and the detailed pharmacological understanding of the neuromuscular junction.[9] In general, short-chain 3FTx members of this group bind muscle nAChRs only, and long-chain members bind both muscle and neuronal receptors. This 3FTx group is sometimes referred to as the "curaremimetic" toxins due to the similarity of their effects with the plant alkaloid curare.[1]
Other groups of 3FTx proteins also bind to different nAChR subtypes; for example, kappa-neurotoxins, which are long-chain dimers, bind neuronal nAChRs, and haditoxin, which is a short-chain dimer, binds both muscle and neuronal subtypes. Non-conventional 3FTx proteins also often bind nAChRs; these were thought to be weaker toxins when first discovered, but the class has been found to possess a range of binding affinities.[1] Recently, a new class of nAChR antagonist 3FTx proteins called omega-neurotoxins has been described.[10]
Muscarinic acetylcholine receptors
A smaller class of 3FTx proteins binds instead to muscarinic acetylcholine receptors, a family of G-protein-coupled receptors. Muscarinic toxins can be either receptor agonists or receptor antagonists, and in some cases the same 3FTx protein is an agonist at one receptor subtype and an antagonist at another. Muscarinic toxins are generally of the short-chain type.[1]
Acetylcholinesterase
A class of 3FTx proteins called fasciculins bind the enzyme acetylcholinesterase and inhibit its activity by blocking access of acetylcholine to the enzyme's active site, thereby preventing acetylcholine breakdown. This class derives its name from its clinical effect, causing muscle fasciculations.[1][11]
Cardiac targets
The second-largest class of 3FTx proteins causes toxicity in cardiac myocytes and can cause increased heart rate and eventually cardiac arrest. These cardiotoxins also often have generalized cytotoxic effects and are sometimes known as cytolysins. The protein targets in myocytes are not generally known for this class, though some members may cause physical damage to the cell by establishing pores in the cell membrane.[1]
Another class, called the beta-cardiotoxins, causes decreased heart rate and are thought to function as beta blockers, antagonists for the beta-1 and beta-2 adrenergic receptors.[1][12]
Less common targets
There are known 3FTx proteins that target a variety of additional protein targets to exert their toxic effects. For example, L-type calcium channels are targeted by calciseptine and platelet aggregation is inhibited via interactions with adhesion proteins by dendroaspin and related proteins.[1] In some cases no toxicity is observed as a result of the 3FTx-target interaction; for example, the mambalgin family of 3FTx proteins interacts with acid-sensing ion channels to produce analgesia without apparent toxic effect in laboratory tests.[13]
Orphan 3FTx proteins
Bioinformatics-based surveys of known protein sequences have often identified a number of sequences likely to form a 3FTx protein structure but whose function has not been experimentally characterized. Thus, it is not known whether these "orphan" proteins are in fact toxins or what their cellular targets might be.[1][14] Genomics studies of gene expression in snakes have shown that members of protein families traditionally considered toxins are widely expressed in snake body tissues and that this expression pattern occurs outside the highly venomous superfamily Caenophidia.[15]
2.2. Structure-function activity relationships
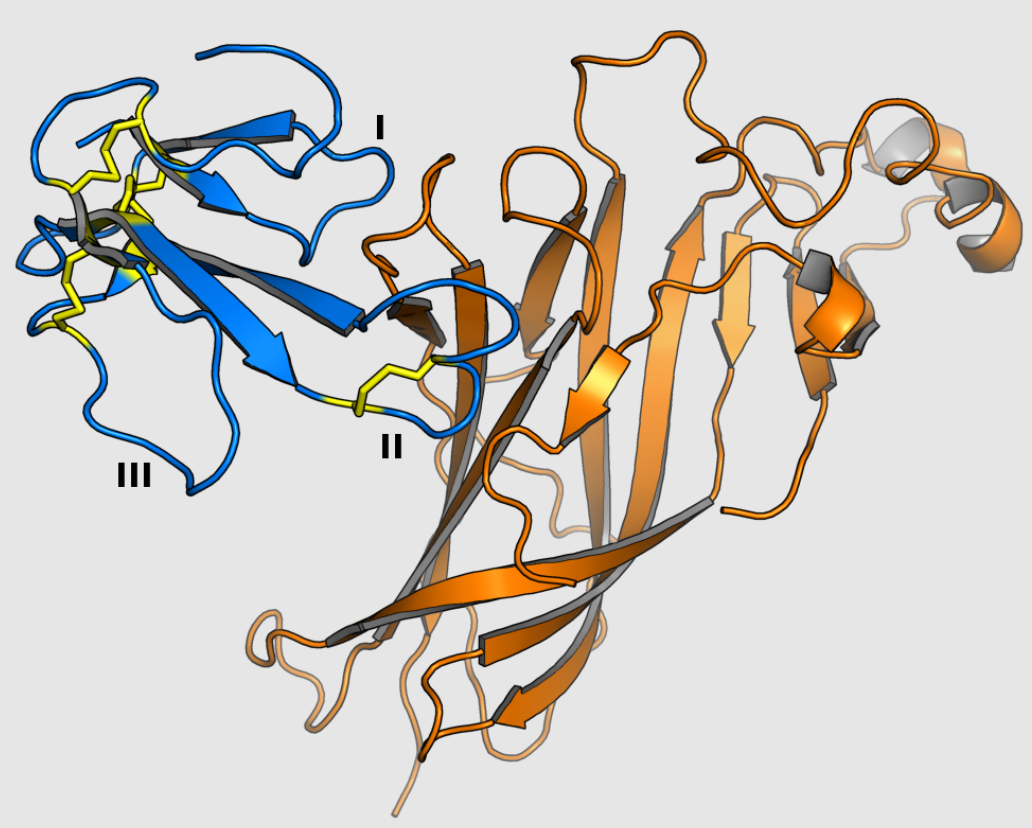
Because 3FTx proteins of similar structure bind a diverse range of cellular protein targets, the relationships between 3FTx protein sequence and their biological activity have been studied extensively, especially among the alpha-neurotoxins. Known functional sites conferring binding affinity and specificity are concentrated in the loops of 3FTx proteins.[1] For example, the crystal structure of alpha-bungarotoxin in complex with the extracellular domain of the alpha-9 nAChR subunit indicates a protein-protein interaction mediated through loops I and II, with no contacts formed by loop III.[16] Interaction surfaces have been mapped for a number of toxins and vary in which loops participate in binding;[1] erabutoxin A uses all three loops to bind nAChRs,[17] while the dendroaspin interaction with adhesion proteins is mediated by three residues in loop III.[18] In some 3FTx proteins with a C-terminal extension, these residues also participate in forming key binding interactions.[1]
The cardiotoxin/cytolysin 3FTx subgroup has a somewhat different set of functionally significant residues due to its distinct mechanism of action, likely involving interactions with phospholipids in the cell membrane,[19] as well as possible functionally significant interactions with other cell-surface molecules such as glycosaminoglycans.[20] A hydrophobic patch of residues contiguous in tertiary structure but distributed over all three loops has been identified as functionally significant in combination with a set of conserved lysine residues conferring local positive charge.[1]
Because of their structural similarity and functional diversity, 3FTx proteins have been used as model systems for the study of protein engineering.[21] Their high binding specificity against targets of pharmacological interest, lack of enzymatic activity, and low immunogenicity have also prompted interest in their potential as drug leads.[22][23][24]
3. Evolution
Although three-finger proteins in general are widely distributed among metazoans, three-finger toxins appear only in snakes.[15][24] They are usually considered to be restricted to the Caenophidia lineage (the taxon containing all venomous snakes), though at least one putative 3FTx homolog has been identified in the genome of the Burmese python, a member of a sister taxon.[15] Early work in analyzing protein homology by sequence alignment in the 1970s suggested 3FTx proteins may have evolved from an ancestral ribonuclease;[25] however, more recent molecular phylogeny studies indicate that 3FTx proteins evolved from non-toxic three-finger proteins.[14][26][27]
Among venomous snakes, the distribution of 3FTx proteins varies; they are particularly enriched in venom from the family Elapidae.[24] [28]In the king cobra (Ophiophagus hannah)[29] and Eastern green mamba (Dendroaspis angusticeps),[30] 3FTx proteins make up about 70% of the protein toxins in venom; in the desert coral snake (Micrurus tschudii) the proportion is reported as high as 95%.[31]
Genes encoding three-finger toxins are thought to have evolved through gene duplication.[26] Traditionally, this has been conceptualized as repeated events of duplication followed by neofunctionalization and recruitment to gene expression patterns restricted to venom glands.[26][29][32] However, it has been argued that this process should be extremely rare and that subfunctionalization better explains the observed distribution.[33] More recently, non-toxic 3FP proteins have been found to be widely expressed in many different tissues in snakes, prompting the alternative hypothesis that proteins of restricted expression in saliva were selectively recruited for toxic functionality.[15] There is evidence that most types of 3FTx proteins have been subject to positive selection (that is, diversifying selection) in their recent evolutionary history,[34] possibly due to an evolutionary arms race with prey species.[27][29] Notable exceptions are the dimeric kappa-bungarotoxin family, likely as a result of evolutionary constraints on the dimer interface, and the cardiotoxin/cytotoxin family, in which a larger fraction of the protein's residues are believed to have functional roles.[34]
The content is sourced from: https://handwiki.org/wiki/Biology:Three-finger_toxin
References
- "Structure, function and evolution of three-finger toxins: mini proteins with multiple targets". Toxicon 56 (6): 855–67. November 2010. doi:10.1016/j.toxicon.2010.07.010. PMID 20670641. https://dx.doi.org/10.1016%2Fj.toxicon.2010.07.010
- Hegde, Raghurama P.; Rajagopalan, Nandhakishore; Doley, Robin; Kini, Manjunatha (2010). "Snake venom three-finger toxins". in Mackessy, Stephen P.. Handbook of venoms and toxins of reptiles. Boca Raton: CRC Press. pp. 287–302. ISBN 9781420008661.
- "Snake three-finger toxin family". http://venomzone.expasy.org/by_species/1856.html.
- "Crystal structure of kappa-bungarotoxin at 2.3-A resolution". Biochemistry 33 (44): 13147–54. November 1994. doi:10.1021/bi00248a026. PMID 7947721. https://dx.doi.org/10.1021%2Fbi00248a026
- "Irditoxin, a novel covalently linked heterodimeric three-finger toxin with high taxon-specific neurotoxicity". FASEB Journal 23 (2): 534–45. February 2009. doi:10.1096/fj.08-113555. PMID 18952712. https://dx.doi.org/10.1096%2Ffj.08-113555
- "Structural and functional characterization of a novel homodimeric three-finger neurotoxin from the venom of Ophiophagus hannah (king cobra)". The Journal of Biological Chemistry 285 (11): 8302–15. March 2010. doi:10.1074/jbc.M109.074161. PMID 20071329. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=2832981
- "Naturally occurring disulfide-bound dimers of three-fingered toxins: a paradigm for biological activity diversification". The Journal of Biological Chemistry 283 (21): 14571–80. May 2008. doi:10.1074/jbc.M802085200. PMID 18381281. https://dx.doi.org/10.1074%2Fjbc.M802085200
- de la Rosa, Guillermo; Olvera, Felipe; Archundia, Irving G.; Lomonte, Bruno; Alagón, Alejandro; Corzo, Gerardo (December 2019). "Horse immunization with short-chain consensus α-neurotoxin generates antibodies against broad spectrum of elapid venomous species" (in en). Nature Communications 10 (1): 3642. doi:10.1038/s41467-019-11639-2. ISSN 2041-1723. PMID 31409779. Bibcode: 2019NatCo..10.3642D. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=6692343
- "Three-finger α-neurotoxins and the nicotinic acetylcholine receptor, forty years on". Journal of Pharmacological Sciences 94 (1): 1–17. January 2004. doi:10.1254/jphs.94.1. PMID 14745112. https://dx.doi.org/10.1254%2Fjphs.94.1
- "A Distinct Functional Site in Ω-Neurotoxins: Novel Antagonists of Nicotinic Acetylcholine Receptors from Snake Venom". ACS Chemical Biology 10 (12): 2805–15. December 2015. doi:10.1021/acschembio.5b00492. PMID 26448325. https://dx.doi.org/10.1021%2Facschembio.5b00492
- "Fasciculins, anticholinesterase toxins from the venom of the green mamba Dendroaspis angusticeps". Journal de Physiologie 79 (4): 232–40. 1984-01-01. PMID 6530667. http://www.ncbi.nlm.nih.gov/pubmed/6530667
- "Beta-cardiotoxin: a new three-finger toxin from Ophiophagus hannah (king cobra) venom with beta-blocker activity". FASEB Journal 21 (13): 3685–95. November 2007. doi:10.1096/fj.07-8658com. PMID 17616557. https://dx.doi.org/10.1096%2Ffj.07-8658com
- "Black mamba venom peptides target acid-sensing ion channels to abolish pain". Nature 490 (7421): 552–5. October 2012. doi:10.1038/nature11494. PMID 23034652. Bibcode: 2012Natur.490..552D. https://dx.doi.org/10.1038%2Fnature11494
- "Molecular evolution and phylogeny of elapid snake venom three-finger toxins". Journal of Molecular Evolution 57 (1): 110–29. July 2003. doi:10.1007/s00239-003-2461-2. PMID 12962311. Bibcode: 2003JMolE..57..110F. https://dx.doi.org/10.1007%2Fs00239-003-2461-2
- "Expression of venom gene homologs in diverse python tissues suggests a new model for the evolution of snake venom". Molecular Biology and Evolution 32 (1): 173–83. January 2015. doi:10.1093/molbev/msu294. PMID 25338510. https://dx.doi.org/10.1093%2Fmolbev%2Fmsu294
- "Crystal structures of free and antagonist-bound states of human α9 nicotinic receptor extracellular domain". Nature Structural & Molecular Biology 21 (11): 976–80. November 2014. doi:10.1038/nsmb.2900. PMID 25282151. https://dx.doi.org/10.1038%2Fnsmb.2900
- "Genetic engineering of snake toxins. The functional site of Erabutoxin a, as delineated by site-directed mutagenesis, includes variant residues". The Journal of Biological Chemistry 270 (16): 9362–9. April 1995. doi:10.1074/jbc.270.16.9362. PMID 7721859. https://dx.doi.org/10.1074%2Fjbc.270.16.9362
- "The effect of the single substitution of arginine within the RGD tripeptide motif of a modified neurotoxin dendroaspin on its activity of platelet aggregation and cell adhesion". Cell Communication & Adhesion 13 (3): 171–83. 2006-01-01. doi:10.1080/15419060600726183. PMID 16798616. https://dx.doi.org/10.1080%2F15419060600726183
- "Snake cytotoxins bind to membranes via interactions with phosphatidylserine head groups of lipids". PLOS ONE 6 (4): e19064. April 2011. doi:10.1371/journal.pone.0019064. PMID 21559494. Bibcode: 2011PLoSO...619064K. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3084733
- "Endocytotic routes of cobra cardiotoxins depend on spatial distribution of positively charged and hydrophobic domains to target distinct types of sulfated glycoconjugates on cell surface". The Journal of Biological Chemistry 289 (29): 20170–81. July 2014. doi:10.1074/jbc.M114.557157. PMID 24898246. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4106332
- "Engineering of three-finger fold toxins creates ligands with original pharmacological profiles for muscarinic and adrenergic receptors". PLOS ONE 7 (6): e39166. 2012-06-14. doi:10.1371/journal.pone.0039166. PMID 22720062. Bibcode: 2012PLoSO...739166F. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3375269
- "Proteome analysis of snake venom toxins: pharmacological insights". Expert Review of Proteomics 5 (6): 787–97. December 2008. doi:10.1586/14789450.5.6.787. PMID 19086859. https://dx.doi.org/10.1586%2F14789450.5.6.787
- Saviola, Anthony J.; Peichoto, María E.; Mackessy, Stephen P. (2014-12-01). "Rear-fanged snake venoms: an untapped source of novel compounds and potential drug leads". Toxin Reviews 33 (4): 185–201. doi:10.3109/15569543.2014.942040. ISSN 1556-9543. https://dx.doi.org/10.3109%2F15569543.2014.942040
- "The three-finger toxin fold: a multifunctional structural scaffold able to modulate cholinergic functions". Journal of Neurochemistry 142 Suppl 2: 7–18. August 2017. doi:10.1111/jnc.13975. PMID 28326549. https://dx.doi.org/10.1111%2Fjnc.13975
- Strydom, D. J. (December 1973). "Snake Venom Toxins: The Evolution of Some of the Toxins Found in Snake Venoms". Systematic Zoology 22 (4): 596–608. doi:10.2307/2412964. https://dx.doi.org/10.2307%2F2412964
- "From genome to "venome": molecular origin and evolution of the snake venom proteome inferred from phylogenetic analysis of toxin sequences and related body proteins". Genome Research 15 (3): 403–20. March 2005. doi:10.1101/gr.3228405. PMID 15741511. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=551567
- "Complex cocktails: the evolutionary novelty of venoms". Trends in Ecology & Evolution 28 (4): 219–29. April 2013. doi:10.1016/j.tree.2012.10.020. PMID 23219381. https://dx.doi.org/10.1016%2Fj.tree.2012.10.020
- de la Rosa, Guillermo; Corrales-García, Ligia L.; Rodriguez-Ruiz, Ximena; López-Vera, Estuardo; Corzo, Gerardo (July 2018). "Short-chain consensus alpha-neurotoxin: a synthetic 60-mer peptide with generic traits and enhanced immunogenic properties" (in en). Amino Acids 50 (7): 885–895. doi:10.1007/s00726-018-2556-0. ISSN 0939-4451. PMID 29626299. http://link.springer.com/10.1007/s00726-018-2556-0.
- "The king cobra genome reveals dynamic gene evolution and adaptation in the snake venom system". Proceedings of the National Academy of Sciences of the United States of America 110 (51): 20651–6. December 2013. doi:10.1073/pnas.1314702110. PMID 24297900. Bibcode: 2013PNAS..11020651V. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3870661
- "Toxicovenomics and antivenom profiling of the Eastern green mamba snake (Dendroaspis angusticeps)". Journal of Proteomics 136: 248–61. March 2016. doi:10.1016/j.jprot.2016.02.003. PMID 26877184. https://backend.orbit.dtu.dk/ws/files/126906502/Toxicovenomics_and_antivenom_profiling_of_the_Eastern_green_mamba_postprint.pdf.
- "Venomic Analysis of the Poorly Studied Desert Coral Snake, Micrurus tschudii tschudii, Supports the 3FTx/PLA₂ Dichotomy across Micrurus Venoms". Toxins 8 (6): 178. June 2016. doi:10.3390/toxins8060178. PMID 27338473. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4926144
- "The structural and functional diversification of the Toxicofera reptile venom system". Toxicon. Advancing in Basic and Translational Venomics 60 (4): 434–48. September 2012. doi:10.1016/j.toxicon.2012.02.013. PMID 22446061. https://dx.doi.org/10.1016%2Fj.toxicon.2012.02.013
- "Restriction and recruitment-gene duplication and the origin and evolution of snake venom toxins". Genome Biology and Evolution 6 (8): 2088–95. August 2014. doi:10.1093/gbe/evu166. PMID 25079342. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=4231632
- "Three-fingered RAVERs: Rapid Accumulation of Variations in Exposed Residues of snake venom toxins". Toxins 5 (11): 2172–208. November 2013. doi:10.3390/toxins5112172. PMID 24253238. http://www.pubmedcentral.nih.gov/articlerender.fcgi?tool=pmcentrez&artid=3847720


