Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Anesthesiology
Animal models of cancer may be classified in a variety of ways. Most simply, they are either spontaneous or induced and mammalian or non-mammalian. Alternatively, they may be categorized by the method of inducing cancer occurrence. A xenograft model involves the transplantation of cancer cells from one species (e.g., human) into a host animal of a different species (e.g., mouse).
- cancer
- surgery
- cancer recurrence
- cancer surgery
1. Introduction
In 2020, an estimated 18 million cancer cases were newly diagnosed (excluding nonmelanoma skin cancer), accounting for approximately 10 million cancer related deaths [1]. Surgical resection of solid tumours remains a mainstay of management of >60% tumours because it offers the best chance of cure [2]. Cancer related mortality is rarely caused by the primary tumour itself, but instead results from the metastatic process and consequent organ dysfunction, which accounts for up to 90% of cancer-related deaths [3,4].
The original hypothesis that the anaesthetic technique during primary cancer resection surgery of curative intent might influence the risk of cancer recurrence or later metastasis was first proposed over a decade ago [5]. This included debate around a potential pro-tumorigenic effect of opioids [6]. Subsequently, the question arose whether opioid sparing anaesthesia-analgesia techniques (e.g., regional anaesthesia) and/or Total Intravenous Anaesthesia (TIVA) techniques can reduce the risk of cancer recurrence and improve survival outcomes for primary cancer surgery [7].
To ultimately prove these hypotheses, large prospective randomised-controlled clinical trials are required to establish if a causal relationship exists between anaesthetic techniques and the risk of cancer recurrence following primary cancer surgery. However, pre-clinical laboratory models, primarily in vivo animal models, retain an important role in translational cancer research for several reasons [8]. Firstly, when designing a robust, prospective, randomised clinical trial it is recommended that the hypothesis is underpinned by quality laboratory evidence to support the trial’s rationale. Secondly, animal models allow researchers with limited resources to test numerous hypotheses, within a more realistic time frame than may be expected in the human clinical setting. Thirdly, in vivo models allow investigators to study the pharmacodynamic effect of various anaesthetic, analgesic, and perioperative interventions on a whole-organism model of cancer biology, which may in turn generate new hypotheses. Lastly, evidence emerging from clinical trials is a slow process and dependent on several external factors, including: the ability to recruit trial participants, availability of personnel, environmental and equipment resources, and large scale funding [9]. For example, the emergence of the CCOVID-19 pandemic had a profound negative impact on ongoing clinical trials other than COVID-19 associated trials [10]. Therefore, experimental evidence from in vivo models will continue to play an important role in supporting or refuting cancer treatment hypotheses. The emergence of onco-anaesthesiology as a distinct clinical subspecialty has driven the exploration of translational research utilising animal models of cancer, traditionally undertaken by oncology researchers.
Animal models of cancer may be classified in a variety of ways. Most simply, they are either spontaneous or induced and mammalian or non-mammalian. Alternatively, they may be categorized by the method of inducing cancer occurrence. However, spontaneously occurring cancers may occur in genetically engineered animal strains or such genetic-engineering may be induced following exposure to various carcinogens, so there is some cross-over in these descriptions. Non-mammalian animals such as zebrafish benefit from being high-throughput and low-cost, ideal for molecular investigation and chemical screening studies, however, significant phenotypical differences limit their usefulness for translational research so they will not be discussed further [11]. Table 1 summarizes the commonly utilized animal models of cancer, each of which are described below.
Table 1. Animal models of cancer classification.
| Classification | Description | Example |
|---|---|---|
| Spontaneous companion animals | Spontaneous cancers in household pets | Mammary carcinoma in dogs |
Spontaneous Transgenic
|
Genetically engineered animals with specific mutations the precipitate the development of cancer during their normal lifespan | Mice with a rat C3(1) simian virus 40 large tumour antigen fusion gene |
Induced Transgenic
|
Inducing genetic mutation via environmental triggers that precipitate cancer development | N-butyl-N-(4-hydroxybutyl) nitrosamine exposed mice & Tetracycline induced Cre recombinase gene expression system |
Induced Allograft
|
Transplantation of cancer cells between animals of the same species that may (syngenic) or may not (non-syngenic) be genetically identical | 4T1 mouse cancer cells transplanted into Bagg Albino (BALB/c) mice |
Induced Xenograft
|
Human cancer cells that may be commercially obtained or patient-specific (PDX) transplanted into other animals | Athymic nude mice Severely compromised immunodeficient (SCID) mice |
2. Xenograft Model
A xenograft model (Figure 1) involves the transplantation of cancer cells from one species (e.g., human) into a host animal of a different species (e.g., mouse). This model is immediately constrained because it requires an immunocompromised host animal to prevent immunological rejection of the non-species cancer cells. Transplantation may be ectopic (deposition of cancer cells beneath the skin) or orthotopic (deposition of cancer cells targeted at the organ of interest). Alternatively, cancer cells may be administered intravenously to mimic metastatic spread or the seeding that is thought to occur during solid tumour cancer surgery [12]. Patient-derived xenografts represent an evolution of this approach utilizing transplantation of fresh tumour biopsies obtained directly from patients into immunocompromised mice to create so-called tumour grafts or avatar mice to enable testing and identification of individualized therapies [13,14].
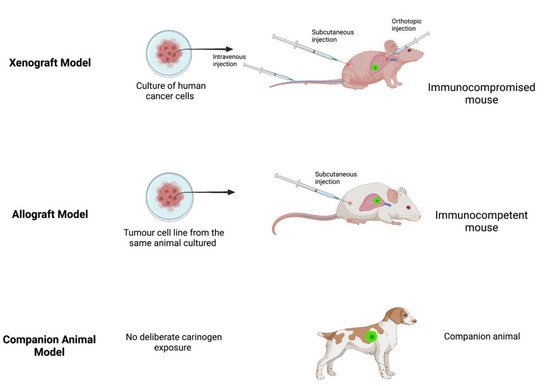
Figure 1. Examples of xenograft, allograft and companion animal models. Created with BioRender.com.
The advantages of xenograft models are that they are relatively inexpensive when using commercially available cancer cell lines and attractive for translational research due to the ability to mimic cancer cell biological traits and the direct evaluation of therapeutic targets in human derived cancer tissue [15,16]. However, several disadvantages arise from the requirement for immunocompromised animals, such as a lack of representative immune response or inflammation, superficial vascularization of the grafts and limited stroma-tumour interactions [15]. Tumour growth rate is variable and often slow, tumour cell composition may not represent the heterogeneity present in the parent cancer and it may not result in metastatic spread, all of which limit the detection of clinically significant metastatic outcomes or falsely increase the perceived efficacy of experimental therapeutic interventions [12,16,17]. The xenograft model also requires quality control because many cell lines have unknown sources or poorly documented receptor expression, and regulatory safeguards are needed to protect researchers from the high communicability risk from handling human cancer tissue [16,18]. Despite these shortcomings, xenograft models have been used extensively to identify potential molecular mechanisms underlying the observed anti-tumour effects of propofol and local anaesthetics as well as pro- and anti-tumour effects following exposure to inhalational anaesthetics [19,20,21,22,23].
This entry is adapted from the peer-reviewed paper 10.3390/medicina58101380
This entry is offline, you can click here to edit this entry!
