Biodiesel industry is expanding rapidly in accordance with the high energy demand and environmental deterioration related to the combustion of fossil fuel. However, poor physicochemical properties and the malperformance of biodiesel fuel still concern the researchers. In this flow, polymers were introduced in biodiesel industry to overcome such drawbacks. This article introduces polymeric biodiesel which is Hydroxyalkanoates methyl ester (HAME) and hydroxybutyrate methyl ester (HBME) that are sourced from carbon-enriched polymers with the help of microbial activity. Composition, production techniques, characteristics, and limitations of polymeric biodiese were explored.
- polymers
- biodiesel
- polyhydroxyalkanoates
- hydroxybutyrate
- Hydroxyalkanoates
1. Introduction
2. Polymeric Biodiesel
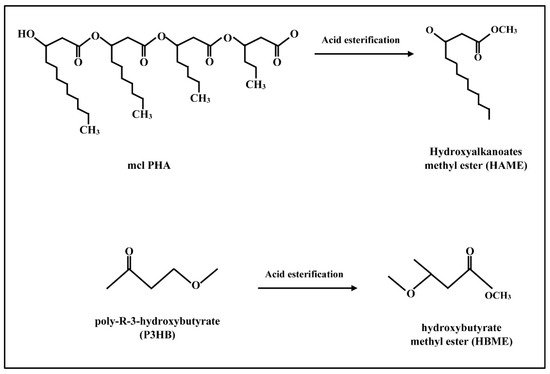
2.1. Production Techniques
| Type of Polymer | Type of PHAs | Source of Carbon | Synthesis Microbe | References |
|---|---|---|---|---|
| Homopolymer | P3HB | Waste glycerol | C. necator DSM 545 | [27] |
| Soy cake and molasses | [28] | |||
| Sugar of coconut, palm, rock, and toddy | C. necator strain A-04 | [29] | ||
| Soybean oil | R. eutropha H16 | [30] | ||
| Pineapple crude glycerol | Bacillus firmus NII 0830 | [31] | ||
| Cooking oil | Burkholderia thailandensis | [32] | ||
| PHHp | Heptanoate | P. putida KTOY06 | [33] | |
| PHV | Undecanoic acid | hydrophila 4AK4 | [34] | |
| PHDD | Sodium dodecanoate | P. putida KT2440 | [35] | |
| PHO | Glycerol and sodium octanoate | P. putida ATCC47054 | [36] | |
| Copolymer | P3HB-co-HA | Gluconate alkanoates | Pseudomonas sp. 61-3 | [37] |
| P3HB-co-P4HB | n-alkanoic acids | R. eutropha H16 | [38] | |
| P3HB-co-HHx | Lauric acid, and oleic acid | A. hydrophila | [39] | |
| P3HB-co-P3HV | Lactose, glucose and galactose | P. hydrogenovora | [40] | |
| P3HB-co-P3HV-co-P3HHx | Dodecanoic acid and propionic acid | Recombinant A. hydrophila 4AK4 | [41] | |
| P(3HP-co-4HB | Glycerol | Recombinant E. coli | [42] |
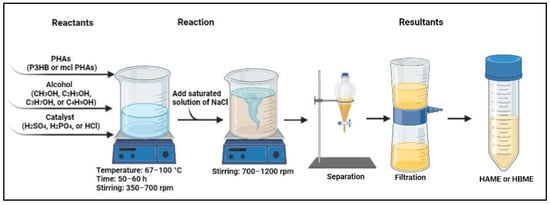
| Polymeric Biodiesel | Polymers Source | Reaction Parameters | Yield (%) |
References | ||||
|---|---|---|---|---|---|---|---|---|
| Alcohol | Catalyst | Ratio % (C in A) * | Temperature (°C) |
Time (h) | ||||
| HAME | mcl PHA | Methanol | H2SO4 | 15 | 100 | 60 | 65 | [24] |
| HAME | mcl PHA | Methanol | H2SO4 | 10 | 67 | 60 | 68 | [49] |
| HBME | P3HB | Methanol | H2SO4 | 15 | 100 | 60 | 52 | [24] |
| HBME | P3HB | Methanol | H2SO4 | 10 | 67 | 60 | 40 | [50] |
| HBME | P3HB | Methanol | H2SO4 | 10 | 67 | 50 | 70.7 | [47] |
| HBME | P3HB | Methanol | H2SO4 | 10 | 67 | 60 | 65 | [48] |
2.2. Characteristics and Limitations
| Physicochemical Properties | Unit | Value |
|---|---|---|
| Density at 20 °C | Kg/m3 | 900 |
| Viscosity 20 °C | mm2/s | 4 |
| Pour point | °C | 1 |
| Flash point | °C | 68.5 |
| Heating value | MJ/kg | 25.1 |
| Cetane number | - | <1 |
| Octane number (RON) | - | 62.2 |
| Oxygen content | %wt | 41 |
| Oxidative stability at 100 °C | h | 8.13 |
This entry is adapted from the peer-reviewed paper 10.3390/polym14193950
References
- Vlnieska, V.; Muniz, A.S.; Oliveira, A.R.; César-Oliveira, M.A.; Kunka, D. Oligocat: Oligoesters as Pseudo-Homogenous Catalysts for Biodiesel Synthesis. Polymers 2022, 14, 210.
- Vlnieska, V.; Muniz, A.S.; Oliveira, A.R.S.; César-Oliveira, M.A.F.; Kunka, D. Synthesis and Chemical Functionalization of Pseudo-Homogeneous Catalysts for Biodiesel Production—Oligocat. Polymers 2022, 14, 19.
- Maafa, I.M. Biodiesel Synthesis from High Free-Fatty-Acid Chicken Fat using a Scrap-Tire Derived Solid Acid Catalyst and KOH. Polymers 2022, 14, 643.
- Zhang, H.; Li, H.; Xu, C.C.; Yang, S. Heterogeneously Chemo/Enzyme-Functionalized Porous Polymeric Catalysts of High-Performance for Efficient Biodiesel Production. ACS Catal. 2019, 9, 10990–11029.
- El-Nahas, A.M.; Salaheldin, T.A.; Zaki, T.; El-Maghrabi, H.H.; Marie, A.M.; Morsy, S.M.; Allam, N.K. Functionalized cellulose-magnetite nanocomposite catalysts for efficient biodiesel production. Chem. Eng. J. 2017, 322, 167–180.
- Nifant’ev, I.; Ivchenko, P. Polymer Cold-Flow Improvers for Biodiesel. Polymers 2021, 13, 1580.
- Nie, S.; Cao, L. Effect of Mixed Commercial Cold Flow Improvers on Flow Properties of Biodiesel from Waste Cooking Oil. Processes 2020, 8, 1094.
- Maehling, F.-O.; Sondjaja, R.; Hess, B.; Couet, J.; Thong, D. Cold Flow Improver with Broad Applicability in Mineral Diesel, Biodiesel and Blends Thereof. CA2899444C, 31 March 2020.
- Muniz, A.S.; Vlnieska, V.; Ferraz, F.A.; Dos Santos Oliveira, A.R.; Ramos, L.P.; César-Oliveira, M.A.F. Polymer additives as cold flow improvers for palm oil methyl esters. Macromol. Symp. 2019, 383, 1800026.
- Xue, Y.; Zhao, Z.; Xu, G.; Lian, X.; Yang, C.; Zhao, W.; Ma, P.; Lin, H.; Han, S. Effect of poly-alpha-olefin pour point depressant on cold flow properties of waste cooking oil biodiesel blends. Fuel 2016, 184, 110–117.
- Baena, L.M.; Zuleta, E.C.; Calderón, J.A. Evaluation of the Stability of Polymeric Materials Exposed to Palm Biodiesel and Biodiesel–Organic Acid Blends. Polymers 2018, 10, 511.
- Haseeb, A.S.M.A.; Masjuki, H.H.; Siang, C.T.; Fazal, M.A. Compatibility of elastomers in palm biodiesel. Renew. Energy 2010, 35, 2356–2361.
- Haseeb, A.S.M.A.; Jun, T.S.; Fazal, M.A.; Masjuki, H.H. Degradation of physical properties of different elastomers upon exposure to palm biodiesel. Energy 2011, 36, 1814–1819.
- Muneer, F.; Rasul, I.; Azeem, F.; Siddique, M.H.; Zubair, M.; Nadeem, H. Microbial Polyhydroxyalkanoates (PHAs): Efficient Replacement of Synthetic Polymers. J. Polym. Environ. 2020, 28, 2301–2323.
- Adeleye, A.T.; Odoh, C.K.; Enudi, O.C.; Banjoko, O.O.; Osiboye, O.O.; Odediran, E.T.; Louis, H. Sustainable synthesis and applications of polyhydroxyalkanoates (PHAs) from biomass. Process Biochem. 2020, 96, 174–193.
- Zhang, X.; Luo, R.; Wang, Z.; Deng, Y.; Chen, G.-Q. Application of (R)-3-Hydroxyalkanoate Methyl Esters Derived from Microbial Polyhydroxyalkanoates as Novel Biofuels. Biomacromolecules 2009, 10, 707–711.
- David, Y.; Joo, J.C.; Yang, J.E.; Oh, Y.H.; Lee, S.Y.; Park, S.J. Biosynthesis of 2-Hydroxyacid-Containing Polyhydroxyalkanoates by Employing butyryl-CoA Transferases in Metabolically Engineered Escherichia coli. Biotechnol. J. 2017, 12, 1700116.
- Riaz, S.; Rhee, K.Y.; Park, S.J. Polyhydroxyalkanoates (PHAs): Biopolymers for Biofuel and Biorefineries. Polymers 2021, 13, 253.
- Cavalheiro, J.M.B.T.; De Almeida, M.C.M.D.; Grandfils, C.; Da Fonseca, M.M.R. Poly(3-hydroxybutyrate) production by Cupriavidus necator using waste glycerol. Process Biochem. 2009, 44, 509–515.
- Oliveira, F.C.; Dias, M.L.; Castilho, L.R.; Freire, D.M.G. Characterization of poly(3-hydroxybutyrate) produced by Cupriavidus necator in solid-state fermentation. Bioresour. Technol. 2007, 98, 633–638.
- Chanprateep, S.; Buasri, K.; Muangwong, A.; Utiswannakul, P. Biosynthesis and biocompatibility of biodegradable poly(3-hydroxybutyrate-co-4-hydroxybutyrate). Polym. Degrad. Stab. 2010, 95, 2003–2012.
- Kahar, P.; Tsuge, T.; Taguchi, K.; Doi, Y. High yield production of polyhydroxyalkanoates from soybean oil by Ralstonia eutropha and its recombinant strain. Polym. Degrad. Stab. 2004, 83, 79–86.
- Althuri, A.; Mathew, J.; Sindhu, R.; Banerjee, R.; Pandey, A.; Binod, P. Microbial synthesis of poly-3-hydroxybutyrate and its application as targeted drug delivery vehicle. Bioresour. Technol. 2013, 145, 290–296.
- Kourmentza, C.; Costa, J.; Azevedo, Z.; Servin, C.; Grandfils, C.; De Freitas, V.; Reis, M.A.M. Burkholderia thailandensis as a microbial cell factory for the bioconversion of used cooking oil to polyhydroxyalkanoates and rhamnolipids. Bioresour. Technol. 2018, 247, 829–837.
- Wang, H.; Li, X.; Chen, G.-Q. Production and characterization of homopolymer polyhydroxyheptanoate (P3HHp) by a fadBA knockout mutant Pseudomonas putida KTOY06 derived from P. putida KT2442. Process Biochem. 2009, 44, 106–111.
- Shen, X.-W.; Yang, Y.; Jian, J.; Wu, Q.; Chen, G.-Q. Production and characterization of homopolymer poly(3-hydroxyvalerate) (PHV) accumulated by wild type and recombinant Aeromonas hydrophila strain 4AK4. Bioresour. Technol. 2009, 100, 4296–4299.
- Hiroe, A.; Watanabe, S.; Kobayashi, M.; Nomura, C.T.; Tsuge, T. Increased synthesis of poly(3-hydroxydodecanoate) by random mutagenesis of polyhydroxyalkanoate synthase. Appl. Microbiol. Biotechnol. 2018, 102, 7927–7934.
- Yang, S.; Li, S.; Jia, X. Production of medium chain length polyhydroxyalkanoate from acetate by engineered Pseudomonas putida KT2440. J. Ind. Microbiol. Biotechnol. 2019, 46, 793–800.
- Matsusaki, H.; Abe, H.; Taguchi, K.; Fukui, T.; Doi, Y. Biosynthesis of poly(3-hydroxybutyrate-co-3-hydroxyalkanoates) by recombinant bacteria expressing the PHA synthase gene phaC1 from Pseudomonas sp. 61-3. Appl. Microbiol. Biotechnol. 2000, 53, 401–409.
- Kimura, H.; Ohura, T.; Takeishi, M.; Nakamura, S.; Doi, Y. Effective microbial production of poly (4-hydroxybutyrate) homopolymer by Ralstonia eutropha H16. Polym. Int. 1999, 48, 1073–1079.
- Lee, S.H.; Oh, D.H.; Ahn, W.S.; Lee, Y.; Choi, J.; Lee, S.Y. Production of poly (3-hydroxybutyrate-co-3-hydroxyhexanoate) by high-cell-density cultivation of Aeromonas hydrophila. Biotechnol. Bioeng. 2000, 67, 240–244.
- Koller, M.; Bona, R.; Chiellini, E.; Fernandes, E.G.; Horvat, P.; Kutschera, C.; Hesse, P.; Braunegg, G. Polyhydroxyalkanoate production from whey by Pseudomonas hydrogenovora. Bioresour. Technol. 2008, 99, 4854–4863.
- Zhao, W.; Chen, G.-Q. Production and characterization of terpolyester poly(3-hydroxybutyrate-co-3-hydroxyvalerate-co-3-hydroxyhexanoate) by recombinant Aeromonas hydrophila 4AK4 harboring genes phaAB. Process Biochem. 2007, 42, 1342–1347.
- Meng, D.; Shi, Z.; Wu, L.; Zhou, Q.; Wu, Q.; Chen, J.; Chen, G. Production and characterization of poly(3-hydroxypropionate-co-4-hydroxybutyrate) with fully controllable structures by recombinant Escherichia coli containing an engineered pathway. Metab. Eng. 2012, 14, 317–324.
- Anjum, A.; Zuber, M.; Zia, K.M.; Noreen, A.; Anjum, M.N.; Tabasum, S. Microbial production of polyhydroxyalkanoates (PHAs) and its copolymers: A review of recent advancements. Int. J. Biol. Macromol. 2016, 89, 161–174.
- Tanadchangsaeng, N.; Pattanasupong, A. Evaluation of Biodegradabilities of Biosynthetic Polyhydroxyalkanoates in Thailand Seawater and Toxicity Assessment of Environmental Safety Levels. Polymers 2022, 14, 428.
- Farag, H.A.; El-Maghraby, A.; Taha, N.A. Optimization of factors affecting esterification of mixed oil with high percentage of free fatty acid. Fuel Process. Technol. 2011, 92, 507–510.
- López-Cuellar, M.R.; Alba-Flores, J.; Rodríguez, J.N.G.; Pérez-Guevara, F. Production of polyhydroxyalkanoates (PHAs) with canola oil as carbon source. Int. J. Biol. Macromol. 2011, 48, 74–80.
- Choonut, A.; Yunu, T.; Pichid, N.; Sangkharak, K. The Optimization Conditions of Polyhydroxybutyrate Methyl Ester from Polyhydroxybutyrate via Acid-Catalyst. Energy Procedia 2017, 138, 435–440.
- Keunun, P.; Rakkarn, T.; Yunu, T.; Paichid, N.; Prasertsan, P.; Sangkharak, K. The Production of Polyhydroxybutyrate by Two-Step Fermentation and the Application of Polyhydroxybutyrate as a Novel Substrate for a Biolubricant. J. Polym. Environ. 2018, 26, 2459–2466.
- Sangkharak, K.; Pichid, N.; Yunu, T.; Srinak, K.; Sornnum, S.; Prasertsan, P. Biofuel production, characterization and degradation of 3-hydroxybutyate methyl ester from polyhydroxybutyrate. Chiang Mai J. Sci 2016, 43, 808–817.
- Wang, S.Y.; Wang, Z.; Liu, M.M.; Xu, Y.; Zhang, X.J.; Chen, G. Properties of a new gasoline oxygenate blend component: 3-Hydroxybutyrate methyl ester produced from bacterial poly-3-hydroxybutyrate. Biomass Bioenergy 2010, 34, 1216–1222.
