Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Chilli pepper, botanically Capsicum annuum L., is an indispensable vegetable cum spice crop grown commercially worldwide for its immature green and red ripe fruits. Universally, the crop is consumed and appreciated for its flavor, colour, aroma, texture, and preserving foods. The crop was probably first used among folks as medicinal plants for its rich bountiful and diversified nutrients, even before it was used in cooking. The understanding on use of such plants helped the masses to sustain since ancient times.
- Capsicum annuum
- capsaicin
- capsaicinoids
- TRPV1 receptor
1. Vitamins
Chillies are known to possess wide range of vitamins especially vitamin A (carotene and C (ascorbic acid), vitamins B2, vitamin B3, vitamin B6, vitamin B9 and vitamin E. Among different vitamins, vitamin C is the only vitamin with strong antioxidant properties that can scavenge free radicals. This happens due to its conjugation of enediol structure for the carbonyl group present in a lactone ring [1]. Such reducing power aids in preventing chronic and degenerative diseases. Pungency in chilli pepper has a directly correlation with ascorbic acid content. Besides, concentration of ascorbic acid increases as the fruit reaches its physiological maturity. Studies revealed that vitamin C has the potentiality in abundance in managing body weight. Vitamin C heals cellular damage, strengthens the immune system and prevents respiratory infections. In a trial among Egyptian chilli genotypes it was found that ascorbic acid content to be high in fresh chillies. Upon drying, chilli loses out most of its vitamin C. The reverse trend was observed for vitamin A upon drying, it increases by 100 times. Vitamin A serves as an anti-inflammatory agent, immunity booster and good retinoid activity. Researchers further revealed in their studies that the level of carotenes and ascorbic acid is six times higher than citrus fruits. In addition, the vitamin C content in red-fruited chillies is twice higher than those of the green fruited ones [2].
2. Nutritional Profile of Chilli Fruits across Species (per 100 g of Edible Portion)
From the below Table 1 it can be well understood that wide array of chilli species provides ample amount of conventional nutrients. Empirical works had indicated on consuming chilli in either fresh or dry form imparts several health benefits [3].
Table 1. Nutritional contents of chilli fruits across species (per 100 g of edible portion).
| Nutrients | Peppers, Hot Chilli, Green (Raw) {a} |
Peppers, Sweet, Green (Raw) {b} | Spices, Pepper, Red or Cayenne {c} | References |
|---|---|---|---|---|
| Carbohydrate (g) | 9.46 | 4.64 | 56.63 | [3][4][5] |
| Protein (g) | 2.00 | 0.86 | 12.01 | [3][4][5] |
| Fat (g) | 0.20 | 0.17 | 17.27 | [3][4][5] |
| Energy (kcal) | 40 | 20 | 318 | [3][4] |
| Iron (mg) | 1.20 | 0.34 | 7.80 | [3][4][5] |
| Calcium (mg) | 18 | 10 | 148 | [3][4][5] |
| Sodium (mg) | 7 | 3 | 30 | [3][4][5] |
| Potassium (mg) | 340 | 175 | 2014 | [3][4][5] |
| Phosphorus (mg) | 46 | 20 | 293 | [3][4] |
| Copper (mg) | 0.30 | 0.066 | 0.373 | [3][4] |
| Selenium (μg) | 0.5 | 0.0 | 8.8 | [3][4] |
{a}—Botanically: C. frutescens. {b}—Botanically: C. annuum. {c}—Botanically: C. frutescens or C. annuum.
3. Phytonutrients and Phytochemical Profiles
In chilli, carotenoids that are yellow-orange-red lipophilic pigments exist in numerous structures, forms at different maturity stages. In chilli fruits, at green colored stage in the chloroplast, an amalgamation of carotenoid and chlorophyll is found. At the intermediary stage of maturity, wide variety of compounds namely, α-carotene, β-carotene, β-cryptoxanthin, zeaxanthin and lutein are seen to be present. De novo, animals aren’t able to fathom carotenoids but can obtain them through daily diets. Carotenoids have the ability to protect bodily tissues from oxidative damages. At red-ripe stage, this carotenoid pigment transforms into capsanthin (6.97 mg/100 g DW), capsorubin (4.50 mg/100 g DW), and 5,6-epoxide (0.79 mg/100 g DW), which produces red colored pigment [6]. In research by Hassan et al. [6], it was revealed that the red color of chilli fruits are induced by a unique group of keto-carotenoids (which includes capsorubin, capsanthin, and cryptocapsin) having κ-ring as end groups. Carotenoids with such κ-ring as end groups have high potential in scavenging oxidative free radicals [7]. Thus, this red color of hot pepper is caused due to carotenoids and is contemplated as one of the ideal sources of β-carotene (28.47 mg/100 g FW), as reviewed by Arimboor et al. [8]. The concentration of phenolic acids, flavonoids in chilli peppers increases as the fruit approaches maturity. In a study by Nagy et al. [9], flavonoids (29.9~37.4 µg/g DW), catechin (6.6~12.3 µg/g DW), derivatives of vanillic acid and narigenin-diglucoside are the most commanding polyphenols in hot peppers. Jeong et al. [10] revealed that flavonoid activity increased at a constant level of ascorbic acid and caffeic acid and has the ability minimize oxidative damage. Red pepper seed and pericarp extracts serves as potent antioxidant agent. Fruit pericarp of chilli pepper has a strong total phenolic (TPC) and flavonoid (TFC) content [11] that scavenges free radicals and can form chelates with ferrous compounds. Besides, in seeds, high scavenging strength is due to ABTS [2,2’-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid)], which works best against superoxide anion radical.
Phytochemicals in chilli peppers especially phenols, quercetin, luteolin and capsaicinoids [12][13][14] serves protection against oxidative stress, diabetes, cancer insurgence, neurological disorders such as Alzheimer’s and Parkinson’s disease [12][15][16]. In a study by researchers on various non-pungent varieties/cultivars ‘California Wonder’ (red pepper), ‘Lamuyo’ (yellow pepper) and ‘Italian Sweet’ (green pepper) exhibited wide span of phenolic classes, which includes 3,7-di-O-α-L-rhamnopyranoside, narigenin-7-O-β-D-(3″-p-coumaroyl)-glucopyranoside and quercetin [17]. In non-pungent chilli pepper genotypes, dihydrocapsiate, capsiate and nordi-hydrocapsiate [18] are present. The composition of phytochemicals varies with genetics, developmental stage and environmental conditions of chilli pepper [19]. Wide array of quercetin are observed in C. annuum, which includes four quercetin glycosides viz. 3-O-rhamnoside-7-O-glucoside and quercetin 3-O-rhamnoside. The quercetin, 3-O-glucoside-7-O-rhamnoside along with rhamnoside-glucoside is bonded at C-3 or C-7 position. Besides, five luteolins had been identified on the pericarp of chilli pepper fruits especially luteolin 6-C-pentoside-8-C-hexoside, luteolin 6-C-hexoside-8-C-pentoside, luteolin 6-C-hexoside, luteolin 8-C-hexoside, luteolin-C-glycosides, luteolin 6,8-di-C-hexoside along with two luteolin-O-glycosides, luteolin 6,8-di-C-hexoside, luteolin (apiosyl-acetyl)-glucoside and luteolin 7-O-(2-apiosyl)-glucoside [19]. Additionally, two apigenin C-glycosides namely apigenin 6,8-di-C-hexoside and apigenin 6-C-pentoside-8-C-hexoside as were also identified in chilli pepper fruits [20]. Furthermore, C-glycosides that were derived from C. annuum L., var. Capel Hot includes luteolin 6-C-glucoside, apigenin 6-C-glucoside-8-C-arabinoside and luteolin 6,8-di-C-glucoside [21].
4. Bioactive Compounds
Wide array of distinct bioactive compounds have been found to be present in different chilli species (Figure 1). These distinct bioactive compounds include capsaicin, homodi-hydrocapsaicin, nordi-hydrocapsaicin, homo-capsaicin and 6,7-dihydrocapsaicin [22]. Chilli pepper fruits vary in their shape, size, color, pungency and flavor and such variation falls out due to genotypic variation, fruit maturity, growing conditions and post-harvest maneuvers. Pungency in chilli depends upon the concentration of capsaicinoids especially capsaicin, which varies in the range of 600 to 13,000 ppm depending upon the genotypes. Capsaicin, the pungent principle in chilli is responsible for causing burning sensation and such irritation can last for hours after ingestion. Other bioactive compounds that are known to be present in chilli include flavonoids and carotenoids. These compounds are known to alleviate and maintain human immune system [23]. Certain principal compounds especially 2-isobutyl-3-methoxypyrazine, linalool, hexanal, trans-2-hexenal, 3-carene and 2, 3-butanedione are known as the constituents of commercial fresh chilli (Capsicum annuum L.) at different maturity stages of the crop [24]. Confirmation on existence of such compounds in chilli was made confirmed by analytical techniques, such as gas chromatography, HPLC, gas chromatography and mass spectrophotometry [2]. Among all these capsaicinoid compounds mentioned above, capsaicin, which is chemically 8-methyl-N-vanillyl-6-nonenamide occupies 80–90% of the total capsaicinoid group and is considered as ‘sine qua non’ factor of the genus Capsicum. The antimicrobial and antioxidant properties of such compounds have drawn interest of both academic and industrial research.
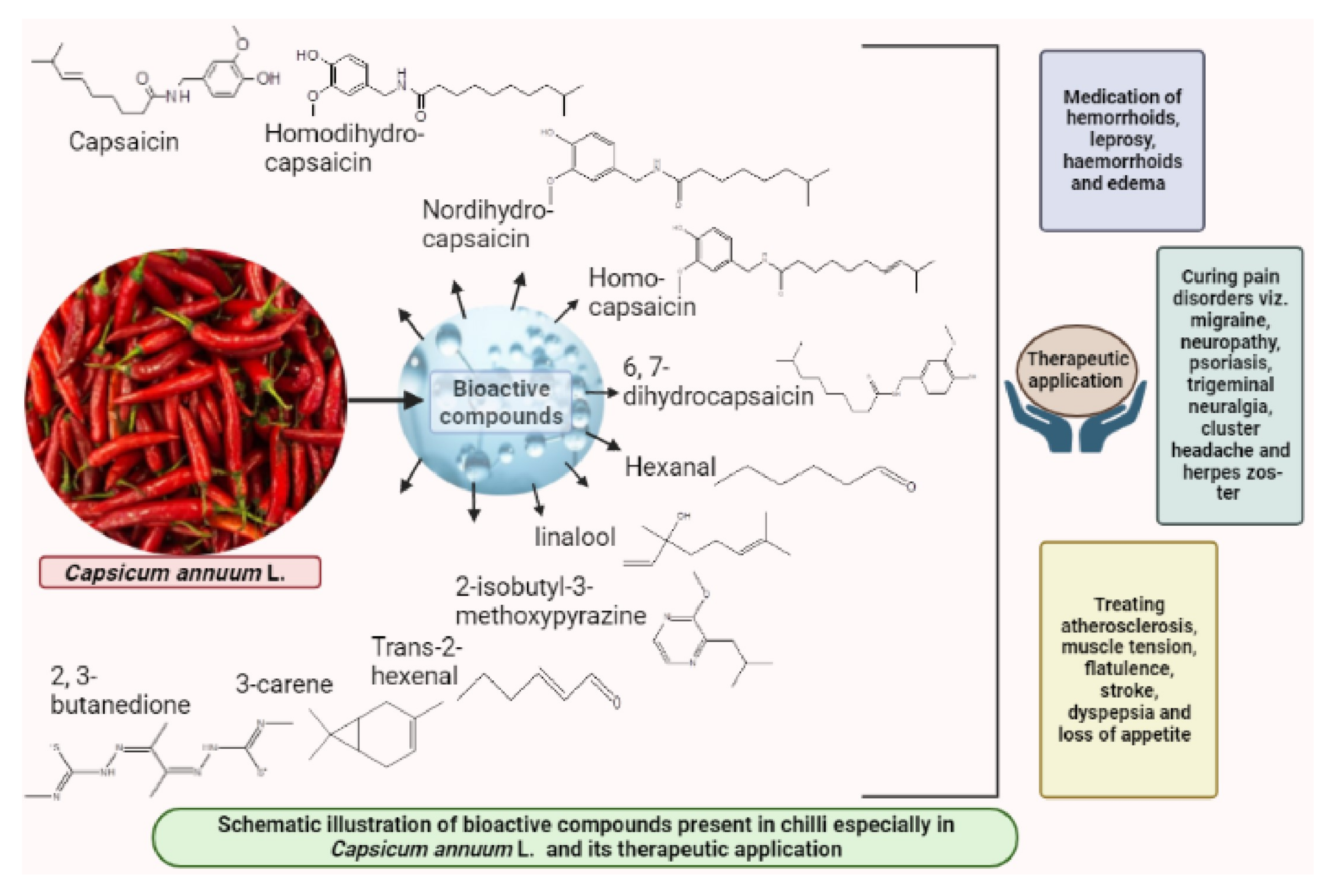
Figure 1. Bioactive compounds present in chilli, their structure and therapeutic applications.
5. Overview on Antioxidant Activities
Antioxidant, a term in general, defined as aniota that has the capacity to delay or prevent the significant way of oxidation of easily oxidizable materials, such as fats or lipids [25][26][27][28]. Not only the lipids that undergo oxidation but it has also been well documented that DNA and protein are very much prone to oxidation [29][30]. In short, antioxidants are the substances that are proficient enough to repair systems. Such system comprises of Fe-transporting proteins that reacts especially with oxidizing agents by inhibiting a specific oxidizing enzyme. Having said that, there is no apt definition worldwide to the term “antioxidant”. Scientists have great deal of attention in plant-derived antioxidants. Capsaicin has the ability to prevent or delay the substrate from getting oxidized [31]. Such antioxidant potentiality of capsaicin was justified later wards by Oyagbemi et al. [27]. The research revealed that capsaicin can prevent carcinogenesis by blocking the translocation of activator protein-1 (AP-1), activator and signal transducer of the transcription (STAT 3), nuclear factor kappa β (NF-kβ). Besides, through generation of ROS capsaicin can arrest further cell cycle and induce apoptosis [27]. Studies have further revealed that capsaicin is potential enough to impede radiation-induced biochemical transformations. Such alterations include lipid peroxidation and oxidation of protein [32], which suggests that physiologically capsaicin can serve as ‘radio-protector’. As mentioned earlier, capsaicin has ability to counteract effects of Reactive Oxygen Species that are associated with cellular metabolic processes causing neurological disorders, cancer, diabetes and cardiovascular disorders [33]. By consuming chilli pepper, detoxification of ROS is possible by several enzymes present in dietary components.
The antioxidant properties in chilli can be attributed and judged by presence of several assaying systems such as dimethyl-4-phenylenediamine assay (DMPD), 2, 2-diphenyl-1-picrylhydrazyl assay (DPPH), 2, 2′-azino-bis[3-ethylbenzothiazoline-6-sulphonic acid] assay (ABTS), Ferric ion reducing antioxidant power assay (FRAP), Cupric reducing antioxidant capacity assay (CuPRAC) [34]. Furthermore, vitamin C, E, anthocyanin and phenolic compounds in chilli pepper possess antioxidant property [35][36]. Phenolic compounds in chilli donate H atoms that neutralize and scavenge radicals inside the body [36]. Medina-Juarez et al. [37] reported that there exists an interdependence between phenolic content and antioxidant potential in chilli pepper. Castro-Concha et al. [34] reported higher levels of carotenoids, ascorbate and glutathione attributed to higher antioxidant activity in Capsicum chinense. Such antioxidants, glutathione and ascorbate contribute to Halliwell Asada Cycle, which detoxifies H2O2 produced in chloroplast without aid of catalase enzyme [38]. According to the findings of [39] capsaicin has been reported to provide synergistic effect with certain therapeutic drugs especially 5-fluorouracil (5-FU). Such drug enhances sensitivity to cholamgiocarcinoma (CCA), a multi-drug resistance type that suppresses cancer cell growth [40][41][42]. The antioxidant activities of capsaicin in chilli are comparable to the synthetic antioxidants especially butylatedhydroxyl toluene (BHT) and butylated hydroxyl anisole (BHA) [41]. Furthermore, intervention studies of capsaicin for both pure capsaicin and microemulsions exhibited higher inhibitory potentialities than synthetic BHT [43]. Such formulations can preferably be used as natural preservatives in meat.
6. Anti-Cancerous Perspectives
Phytochemicals especially capsaicin present in chilli can reduce carcinogenesis and trigger apoptosis in cancerous cells especially in the tissues of skin, colon, bladder, breast, prostate and lung [44][45][46]. Reports revealed that capsaicin has far-reaching anti proliferative activity on human prostate cell in culture. It may act mainly in two different ways especially in case of prostate cancer, direct and indirect pathways. Direct pathway is aided through (i) engendered destruction of primary prostate cancer cells (ii) holding back the expression of prostate–specific antigen (PSA) and (iii) Repression of PSA transcription, which aids in plunge of PSA levels [47]. Another direct pathway of capsaicin that retards the cancer cell growth is antagonistic effect of coenzyme Q controlling the electron transport. Indirect pathway is mediated through interaction of capsaicin with TRPV1 receptor cells resulting in accumulation of Ca2+ cations into the cancerous cells leading to production of apoptosis [46]. Thus, role of Ca2+ at intracellular level have considerable effect on cancer cells including TRP channels leading to intensification of amount of ROS, apoptosis in cancer cells and depolarization of mitochondrial membrane [46][48]. Studies reported that capsaicin can choke migration of breast cancer cell and its metabolites. Metabolites such as phenoxy radicals, which are highly reactive in nature, can even attack DNA molecules and stir up mutagenicity and fatal transformation [49]. Other line of evidence revealed that capsaicin can inhibit nuclear factor-kappa aka NF-κ activation along with tumor necrosis factor-alpha aka TNF-α in prostate cancer cells [47]. Similar anticancerous effects were observed under different conditions pertaining to hepato-cellular carcinoma [50], colon cancer [44], leukemia [46], gastric cancer [48][49] and lung cancer [51] (Table 2). Fruit extracts of Capsicum chinense when applied to cell lines of HepG2 exhibited inhibition of proliferation of hepatocellular carcinoma [52]. Capsaicin can actually bind with ATP generating coenzyme Q and thereby inhibits electron flow in mitochondria through generation of ROS and triggering apoptosis [53]. This ROS thus generated helps in inducing apoptosis pathways and disrupt mitochondrial membrane in pancreatic cancer cells [54]. Research revealed that capsaicin can drastically reduce the seizure alongside migration of cholangiocarcinoma HuCCT1 cells by disabling NF-κB/p65 signaling pathway including expression of MMP-9 [53][54]. Inhibition of nuclear factor kappa-[light-chain-enhancer of activated] B cells (NF-κB) network by capsaicin can simultaneously minimize carcinogenic affects and escalate apoptosis [55][56][57][58]. In a study, Zhang et al. [59] demonstrated that capsaicin through stimulation of TRPV1 can block nuclear relocation of proliferating cell (nuclear) antigen, thus it proves that relation between capsaicin and TRPV1 could be a viable option in bladder cancer treatment. Lin et al. [60] reported that capsaicin can be used in triggering the apoptosis pathway in human κB cancer cells via agitation of mitochondrial membrane including caspase signaling network. Studies revealed that on consuming capsaicin orally can lower down pancreatic cancer, while working in mice [56] and upon consuming capsaicin in diet for 16 months reports revealed that tumor rates were lower particularly in liver cancer cells [50]. Studies further revealed that capsaicin can repress chemically induced skin cancers in mouse models [57][61][62]. Studies related to skin cancer revealed that capsaicin can down-regulate Bcl-2 (B-cell lymphoma 2) expression and bringing about autophagy in B16-F10 lines (acquired from Mus musculus skin melanoma) melanoma cells [63][64][65]. Human cells upon culture with exogenous capsaicin exhibited autophagy [66] apoptosis and inhibition of cell metabolism [67][68][69]. Further experiments conducted by [70][71] by using acquired human cutaneous squamous cell carcinoma (SCC) lines revealed that capsaicin has therapeutic and prophylactic potential in inducing apoptosis and modulating the epidermal growth factor receptor (EGFR) in skin cancers [72].
Table 2. Inhibition effects of capsaicinoids on diversified cells satirizing the antitumor activity.
| Type of Cancer | Diversified Cell Lines | Inhibitory Effects | References |
|---|---|---|---|
| Pancreatic cancer | BxPC-3 and AsPC-1 (pancreatic cancer) | Inhibitory effects by generation of ROS resulting in induction of apoptosis | [73] |
| Blood leukemia | Human myelocytic leukemia (HL-60) | Inhibitory effects by induction of autophagy by caspase-3-dependent process | [46] |
| Human KB cancer | KB (which is derived from HeLa cell line) | Inhibitory effects by staggering cell cycle at G2/M phase causing inducing apoptosis | [60] |
| Tongue cancer | Squamous-Cell Carcinoma (SCC-4) | Inhibitory effects by mitochondria dependent and independent mechanisms causing induction of apoptosis | [71] |
| Lung cancer | NCI-H69, NCI-H82 | Inhibitory effects by arresting cell cycle at GI | [74] |
| Nasopharyngeal cancer | Nasopharyngeal Carcinoma (NPC-TW 039) in human | Inhibitory effects by mitochondrial alteration and stress in endoplasmic reticulum causing induction of apoptosis | [75] |
| Hepatic cancer | HepG2 (human hepatoma) | Inhibitory effects by disruption of ROS causing induction of apoptosis | [76] |
In addition, capsaicin has proved to induce apoptosis through oxidative stress in leukemia cells [59]. Furthermore, in vitro studies revealed that capsaicin can inhibit the growth of blood cancer by inhibiting human T-cell leukemia virus type 1 transcriptional transactivator aka Tax-proteins followed by increasing NF-κB inhibitor with α triggering apoptosis by blocking cell cycle. This substantiates the role of capsaicin as a chemopreventive drug against leukemia [58][77]. A study by Amantini et al. [69] reported that capsaicin has potential effect in treating glioma cancers cells of brain by binding potential TRVP1 in glioma cells triggering autophagy by down-regulating Bcl-2 expression. Research round the world also depicted that capsaicin can impose inhibitory effects on cancerous cells in digestive systems, tongue cancers by inducing the expression of caspase-3 and caspase-9 activities [71][78]. Capsaicin can also mediate ROS activity and induce apoptosis in pancreatic cancer cells suggesting potential use of chilli in treating pancreatic cancer [73]. Lee et al. [77] reported that cell proliferation in colorectal cancer cell lines can be suppresses by capsaicin by restricting the expression of transcription factor 4 (TCF 4) and inhibiting interplay of β-catenin and TCF-4. In addition, capsaicin has the potentiality to lessen the risk of lung cancer in both cancer cell experiments and mouse models by holding back response of E2F (a transcription factor gene in higher eukaryotes) and genetic expression, which discourages its proliferation [74]. In mouse models, capsaicin minimizes the risk of cancer by stabilizing mitochondrial related enzymes using benzopyrene [77][78]. Furthermore; the anticancerous property of capsaicin is also seen in nasopharyngeal related carcinoma by inducing apoptosis mechanisms in NPC lines as reported by Ip et al. [75].
7. Anti-Obesity Activities
Worldwide, obesity is recognized as complex medical condition due to its predominant effects on finance, morbidity and mortality. The disease is the result of interaction effects of sedentary lifestyle, environmental, genetic and dietary effects leading to increased body fat mass. In recent past years, prevalence of obesity has been reported to be the highest in the age category ranging from 50–59 years, revealing an overweight of 60.2% of the population in Malaysia [79]. Alongside the adult population, over the years such increasing trend is observed in teenage group as well. In latest study by NHMS, one in every two adults in Malaysia was obese and females ranked in the highest category [79]. Recently a study by [24], C. annuum contributed to thermogenesis, on application of capsaicin on 3T3-L1 adipocytes lipid content at intracellular level was found to be decreased and thus involved in thermogenesis. In a study by Kang et al. [41], it was affirmed that capsaicin in dietary form could lessen the number of liposaccharides (LPS) content and high-fat diet provoked CLGI related anti-obesity factors. They further reported that capsaicin can also lower down metabolic malfunction in obese or diabetic KKAγ by increasing dissemination of adiponectin and its receptor in mice [41]. Lee et al. [77] reported that lipid accumulation was found decreased in epididymal and mesenteric adipose tissue on application of 0.075% capsaicin in high fat induced obese mice. Furthermore, serum level of triglycerides, cholesterol and glucose was decreased on application of capsaicin in mice. Studies also revealed that capsaicin has anti-proliferative activity in preventing the 3T3-L1 pre-adipocyte and down-regulates the transcription factors, especially PPARγ and thus helps in maintaining body weight [39][80]. Van Avesaat et al. [78] reported that capsaicin has the ability to induce satiety in a short time span, which in turn decrease calorie intake leading to weight loss. In a latest study by Baskaran et al. [81] revealed that upon feeding capsaicin (1µM) heightened the thermogenic and adipogenic proteins in brown adipose tissue (BAT) by stimulating SIRT-1/PRDM-16 dependent methods exhilarating anti-obesity effects.
8. Cardiovascular Roles
The cardio-vascular system in human body is comprised of wide range of sensory nerves sensitive to capsaicin, which plays an effective role in managing cardiovascular function by releasing several neurotransmitters especially Calcitonin gene-related peptide aka CGRP and others including substance P. CAPS has potentiality to provide beneficial effects in cardiovascular system. Peng and Li [82] reported that capsaicin can stimulate release of CGRP by activating TRPV1 benefitting cardiovascular function. Studies by Adams et al. [83] reported that dihydrocapsaicin and capsaicin has potentiality to block platelet aggregation and clotting factors VIII and IX. This helps in lowering the incidence of cardiac diseases. Furthermore, capsaicin can insert itself into the plasmalemma by altering fluidity of the membrane and maintaining its ionic permeability [84]. In addition, Harper et al. [85] reported that capsaicin was capable enough to release calcium cations from intracellular platelets containing TRPV1, which contributed successively to ADP triggering platelet aggregation. Further studies revealed that capsaicinoids especially dihydrocapsaicin can very low-density lipoprotein cholesterol aka LDL-C, low-density lipoprotein cholesterol aka LDL-C, plasma cholesterol as well as C-reactive protein aka CRP, inflammatory cytokines such as interleukin 1 beta aka IL-1β, IL-6, tumor necrosis factor-alpha aka TNF-αand triglycerides. Findings further confirmed that through plasma sterol analysis capsaicinoids can lower plasma cholesterol levels thereby reducing cholesterol absorption. Ahuja et al. [86] reported that on consuming chilli fruits by adult men and women regularly for 4 weeks (3 µg per day approximately) can provoke resistance of serum lipoproteins to oxidation. Manjunatha and Srinivasan [87] reported that capsaicin with concentration of 15 µg/kg of body mass of high-fat-fed rats can reduce lipid peroxide and serum total cholesterol level. The antioxidant property of capsaicinoids attributed to elevated HDL levels and can assiduously enhance the CRT i.e., Reverse Cholesterol Transport pathway, resulting in arresting atherosclerosis and simultaneously promoting cholesterol inflow in THP-1 macrophage acquired foam cells [88]. These above-mentioned statements thus prove that anti-oxidant property of capsaicinoids in cardiovascular, coronary heart diseases and others.
9. Anti-Hyperglycemic/Antidiabetic Activities
Capsaicin has a potent role in reducing glucose and insulin levels in humans. By 2025, studies suggested that number of diabetic patients may rise up to 300 million [89] and thus economically efficient treatments are necessary. Chilli pepper behaves as storehouse of new drugs, which includes α-amylase inhibitor and α-glucosidase inhibitor, which are potent enough to impart antidiabetic activity [89]. On consuming chilli, D-glucose absorption in the intestine is lowered, which could further be utilized in post-prandial rise of glucose and carbohydrate degradation [90]. Studies demonstrated that capsaicin has an effect in carbohydrate metabolism [91][92][93][94][95][96][97][98]. Research on mouse models by Kang et al. [99] revealed that capsaicin on altering gene expression can lessen hyperglycemia. The rate of carbohydrate metabolism were also utilized across human and rats to obtain deep insight in judging anti-diabetic effects of chilli. On administration of capsaicin to rat models through the process of thermogenesis reactions lowers blood glucose levels [100]. Besides, C. frutescens also plays pivotal role in increasing blood insulin level in rat models because of affinity of insulin towards glucose [92]. This depicts involvement of C. frutescens in treatment of diabetes. Recent advances in research pertaining to diabetes by [99], it was revealed that receptors involving TRPV1 play a pivotal partin progression of diabetes type 1 and 2. This progression and development of the diabetes is modulated by capsaicin expressing sensory neurons by ablating TRPV1. Such permanent exclusion of TRPV1 induced by capsaicin decreases insulin resistance, islet infiltration and β-cell stress [96][97][98]. This proves that depletion of TRPV1 expressing neurons prevents diabetes, which is genetically predisposed to type 1 diabetes in mice. Fattori et al. [94] further used Zucker diabetic fat (ZDF) rats for studying various aspects of type 2 diabetes in human and revealed that exclusion of TRPV1 by capsaicin expressing sensory fibers in islets of Langerhans decreased plasma glucose levels, increased insulin secretion and enhanced glucose tolerance. Fattori et al. [94] further demonstrated that on consumption of 10 µg of capsaicin per kg of body mass of mouse models could weaken the activation and augmentation of auto-reactive T cells especially in pancreatic lymph nodes (PLNs). This helps in protecting mice from diabetes. The above studies prove that capsaicin heightens TRPV1 activity, which is important in regulating the activity of islets. A study by Weitz et al. [95] revealed that microphages in islet are capable enough to impart immunological destruction of type 1 diabetes and simultaneously helps in bringing about inflammation in type 2 diabetes.
10. Anti-Inflammatory and Pain Relieving Activities
Out of several phytochemicals in chilli, capsaicin has the ability to confer anti-allergic and -inflammatory activities [101]. The pigment anthocyanin also possesses anti-inflammatory activity [97]. Capsaicin can reduce the inflammatory responses provoked by antigens [98] and simultaneously inhibits dissemination of pro-inflammatory cytokines [99]. Such cytokines includes interleukin 1β aka IL-1β and tumor necrosis factor-α aka TNF-α. Capsaicin can induce inflammatory effects in adipose tissues indicating potential applications as an analgesic [102]. Studies revealed that nordihydrocapsiate (a type of capsinoid) and capsaicin can prevent T cell activation at earlier stage, such events includes NF-κB activation [103]. Furthermore, capsaicin can reduce inflammatory heat, side by side, reports revealed its ability to provide pain relief pertaining to noxious hyperalgesia, which controls the secretion of neurotransmitter causing pain [104]. Studies further demonstrate that capsaicin is capable enough in reducing neuropathic pain [103], as a soothing agent for oral mucositis [105] and bladder pain [106].
11. Anti-Microbial Activities
Spice chilli has been widely used in preserving food since time immemorial [107]. Compounds especially capsaicin that imparts antimicrobial activity along with pungency were observed to be present in Capsicum sp. In addition to pungency, anthoscyanin pigment in chilli also possesses antimicrobial activity [108][109]. Several studies revealed antimicrobial activity of chilli against pathogens especially Clostridium sporogenes, Streptococcus pyogenes and B. subtilis, Candida albicans, Escherichia coli, Sarcinalutea, Pseudomonas aeruginosa [107][108][109]. Jones et al. [110] observed bacterial activity of capsaicin against Helicobacter pyroli causing gastric disorders where inhibitory concentration of capsaicin at bare minimum was found to be 10 µg/mL, which was later confirmed by Zeyrek and Oguz (2005) [111]. Zeyrek and Oguz [111] further observed that capsaicin also exhibited inhibitory activity against wide spectrum of microbial strains. Nascimento et al. [112] observed that the extracts of phytochemicals from C. frutescens especially dihydrocapsaicin, capsaicin and chrysoeriolhad antimicrobial activity against S. aureus, E. coli, Enterococcus faecalis, B.subtillis and Klebsiella pneumonia. In another study by Kurita et al. [113], the antimicrobial capacity of capsaicin was judged with the aid of DNA microarray technology where toxic effect against yeast cells were observed and exerts pleiotropic network of drug defiance, which exhibits genes in relation to membrane biosynthesis and osmotic stress.
12. Anti-Clotting Activity
Phytochemicals in chilli have proven healing effects in wide range of diseases. Studies in mouse models unraveled that capsaicin can inhibit platelet coagulation by withstanding clotting factors VIII: C and IX [83][85]. Studies by Wang et al. [114] demonstrated that capsaicin is more effective than aspirin in altering acute pulmonary thromboembolism. Furthermore, capsaicin can prevent platelet formation by stabilizing membranes of RBCs by impeding the enzyme phospholipaseA2 aka PLA2 as reported by Wang et al. [114]. Therefore, capsaicin has the potential to serve as anti-clotting agent [115].
13. Anesthetic Activities
The capsaicinoids (especially capsaicin) have the ability to provide relief to a wide range of pain disorders in human and are used to prevent several clinical situations related to pain, which includes contact allergy, shingles (Herpes zoster), post-mastectomy syndrome, postsurgical neuromas, cluster headaches, urological disorders, diabetic neuropathy, pruritis, and many others [32]. Friedman et al. [116] reported that proliferation of tumor occurs well under in an acidotic environment, which elevates upon interference with TRPV1 antagonists relieving pain symptoms.
Borbiro et al. [117] revealed that on TRPV1 activation by capsaicin piezo proteins can be obstructed. Such obstruction arises due to Ca-dependent incentive of phospholipase Cδ aka PLCδ, which lowers down phosphor inositides. Administration of such phosphoinositides in the cytosol caused by purged patch clamp, which reduces inner current of Piezo channels and thus reverts inactivation [118]. Furthermore, capsaicin has the capacity to trigger non-neural TRPV1, which stimulates the liberation of prostaglandin E2 (PGE2), IL-6 and interleukin (IL-) 8 [119]. In addition, direct pharmacological desensitization of plasma membrane and inactivation of voltage-gated Na+ channels by TRPV-1 receptors can result in impromptu reduction on neuronal responsiveness and excitability. Anand and Bley [120] further added that excessive concentration of capsaicin (than required) to TRPV1 can result in antagonist effect in mitochondrial dysfunction by inhibiting openly electron chain transport. Further findings had reported that capsaicin can interact with nerve endings, which is transgerminal in nature, and release a neurotransmitter called substance P. Substance P is a neuropeptide, which follows amino acid sequence as Arg-Pro-Lys-Pro-Gln-Gln-Phe-Phe-Gly-Leu-Met [RPKPQQFFGLM]). Studies by Yang and Du [121] revealed that capsaicin can stimulate in release of substance P especially in arthritis and after the recurrent application capsaicin can drain out neuron of substance P and prevents its re-accumulation. Furthermore, it has been observed in rheumatoid arthritis (RA) that sensory afferents, which are highly capsaicin sensitive and densely innervate the synovium and the articular capsule. Thus, capsaicin plays a potential role in anesthesia in clinical conditions.
14. Asthma and Rhinitis Treatment
Systematic studies by Van Gerven et al. [122] revealed that capsaicin can employ its therapeutic action TRPV1-substance P, which produces nociceptive signaling pathway in the nasal mucosa. In addition, a recent study had exhibited that capsaicin helps in desensitization of sensory nerves relieving NAR and IR symptoms for approximately 9 months [101]. Further study revealed that such NAR or IR was correlated with an amplified dissemination of substance P levels in nasal secretions and TRPV1 in the nasal mucosa. A study by Van Rijswijk et al. [123] reported intranasal applications of capsaicin in imparting potential medical benefits in rhinitis type of disorders. Capsaicinon application, provokes initial irritation to the area applied but with passage of time it progressively desensitizes the sensory neural fibers lowering nasal hyper-responsiveness.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196380
References
- Lu, J.M.; Lin, P.H.; Yao, Q.; Chen, C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010, 14, 840–860.
- Leung, F.W. Capsaicin-sensitive intestinal mucosal afferent mechanism and body fat distribution. Life Sci. 2008, 83, 1–5.
- Fathima, S.N. A systemic review on phytochemistry and pharmacological activities of Capsicum annuum. Int. J. Pharm. Pharm. Sci. 2015, 4, 51–68.
- Imran, M.; Butt, M.S.; Suleria, H.A.R. Capsicum annuum Bioactive Compounds: Health Promotion Perspectives. In Bioactive Molecules in Food; Springer: Berlin/Heidelberg, Germany, 2018; pp. 1–22.
- Sharma, J.; Sharma, P.; Chaudhary, B. Estimation of proximate composition of selected species of Capsicum (Capsicum annuum and Capsicum chinense) grown in India. Int. J. Pure Appl. Biosci. 2017, 5, 369–372.
- Hassan, M.N.; Yusof, N.A.; Yahaya, A.F.; Rozali, M.N.N.; Othman, R. Carotenoids of capsicum fruits: Pigment profile and health-promoting functional attributes. Antioxidants 2019, 8, 469.
- Maoka, T.; Tokuda, H.; Suzuki, N.; Kato, H.; Etoh, H. Anti-oxidative, anti-tumor-promoting, and anti-carcinogensis activities of nitroastaxanthin and nitrolutein, the reaction products of astaxanthin and lutein with peroxynitrite. Mar. Drugs 2012, 10, 1391–1399.
- Arimboor, R.; Natarajan, R.B.; Menon, K.R.; Chandrasekhar, L.P.; Moorkoth, V. Red pepper (Capsicum annuum) carotenoids as a source of natural food colors: Analysis and stability-A review. J. Food Sci. Technol. 2015, 52, 1258–1271.
- Nagy, Z.; Daood, H.; Ambrózy, Z.; Helyes, L. Determination of polyphenols, capsaicinoids, and vitamin C in new hybrids of chilli peppers. J. Anal. Methods Chem. 2015, 2015, 102125.
- Jeong, S.M.; Kim, S.Y.; Kim, D.R.; Jo, S.C.; Nam, K.C.; Ahn, D.U.; Lee, S.C. Effect of heat treatment on the antioxidant activity of extracts from citrus peels. J. Agric. Food Chem. 2004, 52, 3389–3393.
- Sim, K.H.; Sil, H.Y. Antioxidant activities of red pepper (Capsicum annuum) pericarp and seed extracts. Int. J. Food Sci. Technol. 2008, 43, 1813–1823.
- Oboh, G.; Rocha, J. Distribution and antioxidant activity of polyphenols in ripe and unripe tree pepper (Capsicum pubescens). J. Food Biochem. 2007, 31, 456–473.
- Liu, Y.; Nair, M.G. Capsaicinoids in the hottest pepper Bhut Jolokia and its antioxidant and antiinflammatory activities. Nat. Prod. Commun. 2010, 5, 91–94.
- Si, W.; Man, S.W.; Chenb, Z.; Chung, H.Y. Stability of capsaicinoid content at raised temperatures. Nat. Prod. Commun. 2014, 9, 985–988.
- Blanco-Ríos, A.K.; Medina-Juárez, L.Á.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of the phenolic and oily fractions of different sweet bell peppers. J. Mex. Chem. Soc. 2013, 57, 137–143.
- Salehi, B.; Mishra, A.P.; Shukla, I.; Sharifi-Rad, M.; Contreras, M.; Segura-Carretero, A.; Fathi, H.; Nasrabadi, N.N.; Kobarfard, F.; Sharifi-Rad, J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018, 32, 1688–1706.
- Morales-Soto, A.; Gómez-Caravaca, A.M.; García-Salas, P.; Segura-Carretero, A.; Fernández-Gutiérrez, A. High-performance liquid chromatography coupled to diode array and electrospray time-of-flight mass spectrometry detectors for a comprehensive characterization of phenolic and other polar compounds in three pepper (Capsicum annuum L.) samples. Food Res. Int. 2013, 51, 977–984.
- Sutoh, K.; Kobata, K.; Yazawa, S.; Watanabe, T. Capsinoid is biosynthesized from phenylalanine and valine in a non-pungent pepper, Capsicum annuum L. cv. CH-19 sweet. Biosci. Biotechnol. Biochem. 2006, 70, 1513–1516.
- Materska, M.; Perucka, I. Antioxidant activity of the main phenolic compounds isolated from hot pepper fruit (Capsicum annuum L.). J. Agric. Food Chem. 2005, 53, 1750–1756.
- Wahyuni, Y.; Ballester, A.R.; Sudarmonowati, E.; Bino, R.J.; Bovy, A.G. Metabolite biodiversity in pepper fruits of thirty-two diverse accessions: Variation in health-related compounds and implications for breeding. Phytochemistry 2011, 72, 1358–1370.
- Materska, M.; Piacente, S.; Stochmal, A.; Pizza, C.; Oleszek, W.; Perucka, I. Isolation and structure elucidation of flavonoid and phenolic acid glycosides from pericarp of hot pepper fruit Capsicum annuum L. Phytochemistry 2003, 63, 893–898.
- Deepa, N.; Kaur, C.; George, B.; Singh, B.; Kapoor, H.C. Antioxidant constituents in some sweet pepper (Capsicum annuum L.) genotypes during maturity. LWT-Food Sci. Technol. 2007, 40, 121–129.
- Howard, L.R.; Smith, R.T.; Wagner, A.B.; Villalon, B.; Burns, E.E. Provitamin A and ascorbic acid content of fresh pepper cultivars (Capsicum annuum) and processed jalapeños. J. Food Sci. 1994, 59, 362–365.
- Hsu, C.; Yen, G. Effects of capsaicin on induction of apoptosis and inhibition of adipogenesis in 3T3-L1 Cells. J. Agric. Food Chem. 2007, 55, 1730–1736.
- Hemalatha, N.; Dhasarathan, P. Comparative study on the antimicrobial activity of Capsicum annuum and Capsicum frutescens. Int. J. Ethnomed. Pharmacol. Res. 2013, 1, 142–147.
- Christensen, R.; Kristensen, P.K.; Bartels, E.M.; Bliddal, H.; Astrup, A. Efficacy and safety of the weight-loss drug rimonabant: A meta-analysis of randomised trials. Lancet 2007, 370, 1706–1713.
- Oyagbemi, A.A.; Saba, A.B.; Azeez, O.I. Capsaicin: A novel chemopreventive molecule and its underlying molecular mechanisms of action. Indian J. Cancer 2010, 47, 53.
- Hossain, M.; Brunton, N.; Barry-Ryan, C.; Martin-Diana, A.B.; Wilkinson, M. Antioxidant activity of spice extracts and phenolics in comparison to synthetic antioxidants. Rasayan J. Chem. 2008, 1, 751–756.
- Sun-Hwa, H.; Jung-Bong, K.; Jong-Sug, P.; Shin-Woo, L.; Kang-Jim, C. A comparison of the carotenoid accumulation in Capsicum varieties that show different ripening colours: Deletion of the capsanthin-capsorubin synthase gene is not a prerequisite for the formation of a yellow pepper. J. Exp. Bot. 2007, 58, 3135–3144.
- Krishna, A.G.G.; Lokesh, B.R.; Sugasini, D.; Kancheva, V.D. Evaluation of the antiradical and antioxidant properties of extracts from Indian red chilli and black pepper by in vitro models. Bulg. Chem. Commun. 2010, 42, 62–69.
- Borovsky, Y.; Oren-Shamir, M.; Ovadia, R.; De Jong, W.; Paran, I. The A locus that controls anthocyanin accumulation in pepper encodes a MYB transcription factor homologous to Anthocyanin2 of Petunia. Theor. Appl. Genet. 2004, 109, 23–29.
- Howard, L.R.; Wildman, R.E. Antioxidant Vitamin and Phytochemical Content of Fresh and Processed Pepper Fruit (Capsicum annuum). In Handbook of Nutraceuticals and Functional Foods; Academic Press: Cambridge, MA, USA; CRC Press: Boca Raton, FL, USA, 2006; pp. 165–191.
- Mennen, L.I.; Walker, R.; Bennetau-Pelissero, C.; Scalbert, A. Risks and safety of polyphenol consumption. Am. J. Clin. Nutr. 2005, 81, 326–329.
- Castro-Concha, L.A.; Canche-Chuc, I.; MirandaHam, M.D.L. Determination of antioxidants in fruit tissues from three accessions of habanero pepper (Capsicum chinense Jacq.). J. Mex. Chem. Soc. 2012, 56, 15–18.
- Sun, T.; Xu, Z.; Wu, C.T.; Janes, M.; Prinyawiwatkul, W.; No, H.K. Antioxidant activities of different colored sweet bell peppers (Capsicum annuum L.). J. Food Sci. 2007, 72, S98–S102.
- Foyer, C.H.; Noctor, G. Redox regulation in photosynthetic organisms: Signaling, acclimation, and practical implications. Antioxid. Redox Signal. 2009, 11, 861–905.
- Medina-Juárez, L.Á.; Molina-Quijada, D.M.; Del-Toro-Sánchez, C.L.; González-Aguilar, G.A.; Gámez-Meza, N. Antioxidant activity of peppers (Capsicum annuum L.) extracts and characterization of their phenolic constituents. Interciencia 2012, 37, 588–593.
- Hong, Z.; Zhao, W.; Yin, Z.; Xie, C.; Xu, Y. Capsaicin enhances the drug sensitivity of cholangiocarcinoma through the inhibition of chemotherapeutic-induced autophagy. PLoS ONE 2015, 10, e0121538.
- Lee, M.S.; Kim, C.T.; Kim, Y. Effect of capsaicin on lipid catabolism in 3T3-L1 adipocytes. Phytother. Res. 2011, 25, 935–939.
- Ezekiel, J.A.T.; Oluwole, O.J.A. Effects of capsaicin on coagulation: Will this be the new blood thinner? Clin. Med. Res. 2014, 3, 145–149.
- Kang, M.C.; Kang, N.; Ko, S.C.; Kim, Y.B.; Jeon, Y.J. Anti-obesity effect of seaweeds of Jeju Island on the differentiation of 3T3 L1-preadipocytes and obese mice fed a high fat diet. Food Chem. Toxicol. 2016, 90, 36–44.
- Wang, F.; Zhao, J.; Liu, D.; Zhao, T.; Lu, Z.; Zhu, L.; Cao, L.; Yang, J.; Jin, J.; Cai, Y. Capsaicin reactivates hMOF in gastric cancer cells and induces cell growth inhibition. Cancer Biol. Ther. 2016, 17, 1117–1125.
- Wang, Y.; Liu, B.; Wen, X.; Li, M.; Wang, K.; Ni, Y. Quality analysis and microencapsulation of chilli seed oil by spray drying with starch sodium octenylsuccinate and maltodextrin. Powder Technol. 2017, 312, 294–298.
- Övey, I.S.; Güler, Y. Apoptotic efficiency of capecitabine and 5-fluorouracil on human cancer cells through TRPV1 channels. Indian J. Biochem. Biophys. 2020, 57, 64–72.
- Arora, R.; Gill, N.S.; Chauhan, G.; Rana, A.C. An overview about versatile molecule Capsaicin. Int. J. Pharm. Sci. Drug Res. 2011, 3, 280–286.
- Tsou, M.F.; Lu, H.F.; Chen, S.C.; Wu, L.T.; Chen, Y.S.; Kuo, H.M.; Lin, S.S.; Chung, J.G. Involvement of Bax, Bcl-2, Ca2+ and caspase-3 in capsaicin-induced apoptosis of human leukemia HL-60 cells. Anticancer Res. 2006, 26, 1965–1971.
- Mori, A.; Lehmann, S.; O’Kelly, J.; Kumagai, T.; Desmond, J.C.; Pervan, M.; McBride, W.H.; Kizaki, M.; Koeffler, H.P. Capsaicin, a component of red peppers, inhibits the growth of androgen-independent, p53 mutant prostate cancer cells. Cancer Res. 2006, 66, 3222–3229.
- Huh, H.C.; Lee, S.Y.; Lee, S.K.; Park, N.H.; Han, I.S. Capsaicin induces apoptosis of cisplatin-resistant stomach cancer cells by causing degradation of cisplatin-inducible aurora-A protein. Nutr. Cancer. 2011, 63, 1095–1103.
- Wang, H.M.; Chuang, S.M.; Su, Y.C.; Li, Y.H.; Chueh, P.J. Downregulation of tumor-associated NADH oxidase, tNOX (ENOX2), enhances capsaicin-induced inhibition of gastric cancer cell growth. Cell Biochem. Biophys. 2011, 61, 355–366.
- Amruthraj, N.J.; Raj-Preetam, J.P.; Saravanan, S.; Lebel-Antoine, L. In vitro studies on anticancer activity of capsaicinoids from Capsicum chinense against human hepatocellular carcinoma cells. Int. J. Pharm. Pharm. Sci. 2014, 6, 254–558.
- Anandakumar, P.; Kamaraj, S.; Jagan, S.; Ramakrishnan, G.; Asokkumar, S.; Naveenkumar, C.; Raghunandhakumar, S.; Devaki, T. Capsaicin inhibits benzo (a) pyrene-induced lung carcinogenesis in an in vivo mouse model. Inflamm. Res. 2012, 61, 1169–1175.
- Loizzo, M.R.; Pugliese, A.; Bonesi, M.; Menichini, F.; Tundis, R. Evaluation of chemical profile and antioxidant activity of twenty cultivars from Capsicum annuum, Capsicum baccatum, Capsicum chacoense and Capsicum chinense: A comparison between fresh and processed peppers. LWT-Food Sci. Technol. 2015, 64, 623–631.
- Macho, A.; Blázquez, M.V.; Navas, P.; Muñoz, E. Induction of apoptosis by vanilloid compounds does not require de novo gene transcription and activator protein 1 activity. Cell Death Differ. 1998, 9, 277–286.
- Macho, A.; Calzado, M.A.; Munoz-Blanco, J.; Gomez-Diaz, C.; Gajate, C.; Mollinedo, F.; Navas, P.; Munoz, E. Selective induction of apoptosis by capsaicin in transformed cells: The role of reactive oxygen species and calcium. Cell Death Differ. 1999, 6, 155–165.
- Macho, A.; Lucena, C.; Calzado, M.A.; Blanco, M.; Donnay, I.; Appendino, G.; Munoz, E. Phorboid 20-homovanillates induce apoptosis through a VR1-independent mechanism. Chem. Biol. 2000, 7, 483–492.
- Zhang, R.; Humphreys, I.; Sahu, R.P.; Shi, Y.; Sirivastava, S.K. in vitro and in vivo induction of apoptosis by capsaicin in pancreatic cancer cells is mediated through ROS generation and mitochondrial death pathway. Apoptosis 2008, 13, 1465–1478.
- Han, S.S.; Keum, Y.S.; Seo, H.J.; Chun, K.S.; Lee, S.S.; Surh, Y.J. Capsaicin suppresses phorbol esterinduced activation of NF-κB/Rel and AP-1 transcription factors in mouse epidermis. Cancer Lett. 2001, 164, 119–126.
- Patel, P.S.; Varney, M.L.; Dave, B.J.; Singh, R.K. Regulation of constitutive and induced NF-κB activation in malignant melanoma cells by capsaicin modulates interleukin-8 production and cell proliferation. J. Interferon Cytokine Res. 2002, 22, 427–435.
- Zhang, J.; Nagasaki, M.; Tanaka, Y.; Morikawa, S. Capsaicin inhibits growth of adult T-cell leukemia cells. Leuk. Res. 2003, 27, 275–283.
- Lin, C.; Lu, W.; Wang, C.; Chan, Y.; Chen, M. Capsaicin induces cell cycle arrest and apoptosis in human KB cancer cells. BMC Complement. Altern. Med. 2013, 13, 46.
- Akagi, A.; Sano, N.; Uehara, H.; Minami, T.; Otsuka, H.; Izumi, K. Noncarcinogencity of capsaicinoids in B6C3F1 mice. Food Chem. Toxicol. 1998, 36, 1065–1071.
- Park, K.K.; Surh, Y.J. Effects of capsaicin on chemically-induced two-stage mouse skin carcinogenesis. Cancer Lett. 1997, 114, 183–184.
- Hail, N.; Lotan, R. Examining the role of mitochondrial respiration in vanilloid-induced apoptosis. J. Natl. Cancer Inst. 2002, 94, 1281–1292.
- Hwang, M.K.; Bode, A.M.; Byun, S.; Song, N.R.; Lee, H.J.; Lee, K.W.; Dong, Z. Cocarcinogenic effect of capsaicin involves activation of EGFR signaling but not TRPV1EGFR-dependent cocarcinogenic effect of capsaicin. Cancer Res. 2010, 70, 6859–6869.
- Jun, H.S.; Park, T.; Lee, C.K.; Kang, M.K.; Park, M.S.; Kang, H.I.; Surh, Y.J.; Kim, O.H. Capsaicin induced apoptosis of B16-F10 melanoma cells through down-regulation of Bcl-2. Food Chem. Toxicol. 2007, 45, 708–715.
- Choi, C.H.; Jung, Y.K.; Oh, S. Selective induction of catalase mediated autophagy by dihydrocapsaicin in lung cell lines. Free Radic. Biol. Med. 2009, 49, 245–257.
- Ghosh, A.K.; Basu, S. Fas-associated factor 1 is a negative regulator in capsaicin induced cancer cell apoptosis. Cancer Lett. 2010, 287, 142–149.
- Ito, K.; Nakazato, T.; Yamato, K.; Miyakawa, Y.; Yamada, T.; Hozumi, N.; Segawa, K.; Ikeda, Y.; Kizaki, M. Induction of apoptosis in leukemic cells by homovanillic acid derivative, capsaicin, through oxidative stress: Implication of phosphorylation of p53 at Ser-15 residue by reactive oxygen species. Cancer Res. 2004, 64, 1071–1078.
- Amantini, C.; Mosca, M.; Nabissi, M.; Lucciarini, R.; Caprodossi, S.; Arcella, A.; Giangaspero, F.; Santoni, G. Capsaicin-induced apoptosis of glioma cells is mediated by TRPV1 vanilloid receptor and requires p38 MAPK activation. J. Neurochem. 2007, 102, 977–990.
- Tanaka, T.; Kohno, H.; Sakata, K.; Yamada, Y.; Hirose, Y.; Sugie, S.; Mori, H. Modifying effects of dietary capsaicin and rotenone on 4-nitroquinoline 1-oxide-induced rat tongue carcinogenesis. Carcinogenesis 2002, 23, 1361–1367.
- Ip, S.W.; Lan, S.H.; Huang, A.C.; Yang, J.S.; Chen, Y.Y.; Huang, H.Y.; Lin, Z.P.; Hsu, Y.M.; Yang, M.D.; Chiu, C.F.; et al. Capsaicin induces apoptosis in SCC-4 human tongue cancer cells through mitochondria dependent and independent pathways. Environ. Toxicol. 2012, 27, 332–341.
- Lee, S.H.; Krisanapun, C.; Baek, S.J. NSAID-activated gene-1 as a molecular target for capsaicin induced apoptosis through a novel molecular mechanism involving GSK3β, C/EBPβ, and ATF3. Carcinogenesis 2010, 31, 719–728.
- Pramanik, K.C.; Boreddy, S.R.; Srivastava, S.K. Role of mitochondrial electron transport chain complexes in capsaicin mediated oxidative stress leading to apoptosis in pancreatic cancer cells. PLoS ONE 2011, 6, e20151.
- Brown, K.C.; Witte, T.R.; Hardman, W.E.; Luo, H.; Chen, Y.C.; Carpenter, A.B.; Lau, J.K.; Dasgupta, P. Capsaicin displays antiproliferative activity against human small cell lung cancer in cell culture and nude mice models via the E2F pathway. PLoS ONE 2010, 5, e10243.
- Ip, S.W.; Lan, S.H.; Lu, H.F.; Huang, A.C.; Yang, J.S.; Lin, J.P.; Huang, H.Y.; Lien, J.C.; Ho, C.C.; Chiu, C.F.; et al. Capsaicin mediates apoptosis in human nasopharyngeal carcinoma NPC-TW 039 cells through mitochondrial depolarization and endoplasmic reticulum stress. Hum. Exp. Toxicol. 2012, 31, 539–549.
- Huang, S.P.; Chen, J.C.; Wu, C.C.; Chen, C.T.; Tang, N.Y.; Ho, Y.T.; Lo, C.; Lin, J.P.; Chung, J.G.; Lin, J.G. Capsaicin-induced apoptosis in human hepatoma HepG2 cells. Anticancer Res. 2009, 29, 165–174.
- Lee, G.R.; Shin, M.K.; Yoon, D.J.; Kim, A.R.; Yu, R.; Park, N.H. Topical application of capsaicin reduces visceral adipose fat by affecting adipokine levels in high-fat diet-induced obese mice. Obesity 2013, 1, 115–122.
- Van Avesaat, M.; Troost, F.J.; Westerterp-Plantenga, M.S.; Helyes, Z.; Le Roux, C.W.; Dekker, J.; Masclee, A.A.; Keszthelyi, D. Capsaicin-induced satiety is associated with gastrointestinal distress but not with the release of satiety hormones. Am. J. Clin. Nutr. 2016, 103, 305–313.
- Azlan, A.; Sultana, S.; Huei, C.S.; Razman, M.R. Antioxidant, Anti-Obesity, Nutritional and Other Beneficial Effects of Different Chilli Pepper: A Review. Molecules 2022, 27, 898.
- Mehmet, B.; Metin, Y.; Gulhan, A.; Omer, T.; Oruc, A. Effect of capsaicin on transcription factor in 3T3-L1 cell line. East. J. Med. 2015, 20, 34–45.
- Baskaran, P.; Krishnan, V.; Fettel, K.; Gao, P.; Zhu, Z.; Ren, J.; Thyagarajan, B. TRPV1 activation counters diet-induced obesity through sirtuin-1 activation and PRDM-16 deacetylation in brown adipose tissue. Int. J. Obes. 2017, 41, 739–749.
- Peng, J.; Li, Y.J. The vanilloid receptor TRPV1: Role in cardiovascular and gastrointestinal protection. Eur. J. Pharmacol. 2010, 627, 1–7.
- Adams, M.J.; Ahuja, K.D.K.; Geraghty, D.P. Effect of capsaicin and dihydrocapsaicin on in vitro blood coagulation and platelet aggregation. Thromb. Res. 2009, 124, 721–723.
- Panchal, S.K.; Bliss, E.; Brown, L. Capsaicin in metabolic syndrome. Nutrients 2018, 10, 630.
- Harper, A.G.S.; Brownlow, S.L.; Sage, S.O. A role for TRPV1 in agonist-evoked activation of human platelets. J. Thromb. Haemost. 2009, 7, 330–338.
- Ahuja, K.D.K.; Kunde, D.A.; Ball, M.J.; Geraghty, D.P. Effects of capsaicin, dihydrocapsaicin, and curcumin on copper-induced oxidation of human serum lipids. J. Agric. Food Chem. 2006, 54, 6436–6439.
- Manjunatha, H.; Srinivasan, K. Hypolipidemic and antioxidant effects of curcumin and capsaicin in high-fat-fed rats. Can. J. Physiol. Pharmacol. 2007, 85, 588–596.
- Hu, Y.W.; Ma, X.; Huang, J.L.; Mao, X.R.; Yang, J.Y.; Zhao, J.Y.; Li, S.F.; Qiu, Y.R.; Yang, J.; Zheng, L.; et al. Dihydrocapsaicin attenuates plaque formation through a PPARγ/LXRα pathway in apoe−/−mice fed a high-fat/high-cholesterol diet. PLoS ONE 2013, 8, e66876.
- Sy, G.Y.; Cissé, A.; Nongonierma, R.B.; Sarr, M.; Mbodj, N.A.; Faye, B. Hypoglycaemic and antidiabetic activity of acetonic extract of Vernonia colorata leaves in normoglycaemic and alloxaninduced diabetic rats. J. Ethnopharmacol. 2005, 98, 171–175.
- Kwon, D.Y.; Kim, Y.S.; Ryu, S.Y.; Cha, M.R.; Yon, G.H.; Yang, H.J.; Kim, M.J.; Kang, S.; Park, S. Capsiate improves glucose metabolism by improving insulin sensitivity better than capsaicin in diabetic rats. J. Nutr. Biochem. 2013, 24, 1078–1085.
- Magied, M.M.A.; Salama, N.A.R.; Ali, M.R. Hypoglycemic and hypocholesterolemia effects of intragastric administration of dried red chilli pepper (Capsicum annuum) in alloxan-induced diabetic male albino rats fed with high-fat-diet. J. Food Nutr. Res. 2014, 11, 850–856.
- Islam, M.S.; Choi, H. Dietary red chilli (Capsicum frutescens L.) is insulinotropic rather than hypoglycemic in type 2 diabetes model of rats. Phytother. Res. 2008, 22, 1025–1029.
- Xu, W.; Liu, J.; Ma, D.; Yuan, G.; Lu, Y.; Yang, Y. Capsaicin reduces Alzheimer-associated tau changes in the hippocampus of type 2 diabetes rats. PLoS ONE 2017, 12, e0172477.
- Fattori, V.; Hohmann, M.S.N.; Rossaneis, A.C.; Pinho-Ribeiro, F.A.; Verri, W.A. Capsaicin: Current understanding of its mechanisms and therapy of pain and other pre-clinical and clinical uses. Molecules 2016, 21, 844.
- Weitz, J.R.; Makhmutova, M.; Almaça, J.; Stertmann, J.; Aamodt, K.; Brissova, M.; Speier, S.; Rodriguez-Diaz, R.; Caicedo, A. Mouse pancreatic islet macrophages use locally released ATP to monitor beta cell activity. Diabetologia 2018, 61, 182–192.
- Kim, C.S.; Kawada, T.; Kim, B.S.; Han, I.S.; Choe, S.Y.; Kurata, T.; Yu, R. Capsaicin exhibits anti-inflammatory property by inhibiting IkB-a degradation in LPS-stimulated peritoneal macrophages. Cell. Signal. 2003, 15, 299–306.
- Wang, H.B.; Nair, M.G.; Strasburg, G.M.; Chang, Y.C.; Booren, A.M.; Gray, J.I.; DeWitt, D.L. Antioxidant and antiinflammatory activities of anthocyanins and their aglycon, cyanidin, from tart cherries. J. Nat. Prod. 1999, 62, 294–296.
- Spiller, F.; Alves, M.K.; Vieira, S.M.; Carvalho, T.A.; Leite, C.E.; Lunardelli, A.; Polomi, J.A.; Cunha, F.Q.; Oliveira, J.R. Anti-inflammatory effects of red pepper (Capsicum baccatum) on carrageenan- and antigen-induced inflammation. J. Pharm. Pharmacol. 2008, 60, 473–478.
- Kang, J.H.; Kim, C.S.; Han, I.S.; Kawada, T.; Yu, R. Capsaicin, a spicy component of hot peppers, modulates adipokine gene expression and protein release from obese-mouse adipose tissues and isolated adipocytes, and suppresses the inflammatory responses of adipose tissue macrophages. FEBS Lett. 2007, 581, 4389–4396.
- Okunlola, G.O.; Olatunji, O.A.; Akinwale, R.O.; Tariq, A.; Adelusi, A.A. Physiological response of the three most cultivated pepper species (Capsicum spp.) in Africa to drought stress imposed at three stages of growth and development. Sci. Hortic. 2017, 224, 198–205.
- Sancho, R.; Lucena, C.; Macho, A.; Calzado, M.A.; Blanco-Molina, M.; Minassi, A.; Appendino, G.; Munoz, E. Immunosuppressive activity of capsaicinoids: Capsiate derived from sweet peppers inhibits NF-ĸB activation and is a potent anti-inflammatory compound in vivo. Eur. J. Immunol. 2002, 32, 1753–1763.
- Fraenkel, L.; Bogardus, S.T.; Concato, J.; Wittink, D.R. Treatment options in knee osteoarthritis: The patient’s perspective. Arch. Intern. Med. 2004, 164, 1299–1304.
- Lynn, B. Capsaicin: Action on nocicepetive C-fibres and therapeutic potential. Pain 1990, 41, 61–69.
- Sindrup, S.H.; Jensen, T.S. Efficacy of pharmacological treatments of neuropathic pain: An update and effect related to mechanism of drug action. Pain 1999, 83, 389–400.
- Berger, A.; Henderson, M.; Nadoolman, W.; Duffy, V.; Cooper, D.; Sabersli, L.; Bartoshuk, L. Oral capsaicin provides temporary relief for oral mucositis pain secondary to chemotherapy/radiation therapy. J. Pain Symptom Manag. 1995, 10, 243–248.
- Cruz, F. Mechanisms involved in new therapies for overactive bladder. Urology 2004, 63, 65–73.
- Omolo, M.A.; Wong, Z.Z.; Mergen, A.K.; Hastings, J.C.; Le, N.C.; Reiland, H.A.; Case, K.A.; Baumier, D.J. Antimicrobial Properties of Chilli Peppers. Infect. Dis. Ther. 2014, 2, 145.
- Careaga, M.; Fernández, E.; Dorantes, L.; Mota, L.; Jaramillo, M.E.; Hernandez-Sanchez, H. Antibacterial activity of Capsicum extract against Salmonella typhimurium and Pseudomonas aeruginosa inoculated in raw beef meat. Int. J. Food Microbiol. 2003, 83, 331–335.
- Soetarno, S.; Sukrasno, S.; Yulinah, E.; Sylvia, S. Antimicrobial activities of the ethanol extracts of Capsicum fruits with different levels of pungency. J. Membr. Sci. 1997, 2, 57–63.
- Jones, N.L.; Shabib, S.; Sherman, P.M. Capsaicin as an inhibitor of growth of the gastric pathogen Helicobacter pyroli. FEMS Microbiol. Lett. 1997, 146, 223–227.
- Zeyrek, Y.F.; Oguz, E. Invitro activity of capsaicin against Heylicobacter pylori. Ann. Microbiol. 2005, 55, 125–127.
- Nascimento, P.L.A.; Nascimento, T.C.E.S.; Ramos, N.S.M.; Silva, G.R.; Gomes, J.E.G.; Falcao, R.A.E.; Moreira, K.A.; Porto, A.L.F.; Silva, T.M.S. Quantification, antioxidant and antimicrobial Activity of phenolics isolated from different extracts of Capsicum frutescens (Pimentamalagueta). Molecules 2014, 19, 5434–5447.
- Kurita, S.K.; Kitagawa, E.; Kim, C.H.; Momose, Y.; Iwahashi, H. Studies of the antimicrobial mechanism of Capsaicin using yeast DNA microarray. Biosci. Biotechnol. Biochem. 2002, 66, 532–536.
- Wang, J.P.; Hsu, M.F.; Hsu, T.P.; Teng, C.M. Antihemostatic and antithrombotic effects of capsaicin in comparison with aspirin and indomethacin. Thromb. Res. 1985, 37, 669–679.
- Wang, J.P.; Hsu, M.F.; Teng, C.M. Antiplatelet effect of capsaicin. Thromb. Res. 1984, 36, 497–507.
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Denning, K.L.; Tirona, M.T.; Valentovic, M.A.; Miles, S.L.; Dasgupta, P. Capsaicinoids: Multiple effects on angiogenesis, invasion and metastasis in human cancers. Biomed. Pharmacother. 2019, 118, 109317.
- Borbiro, I.; Badheka, D.; Rohacs, T. Activation of TRPV1 channels inhibits mechanosensitive piezo channel activity by depleting membrane phosphoinositides. Sci. Signal. 2015, 8, ra15.
- Della Pietra, A.; Mikhailov, N.; Giniatullin, R. The emerging role of mechanosensitive Piezo channels in migraine pain. Int. J. Mol. Sci. 2020, 21, 696.
- Salari, M.; Salari, R.; Rafatpanah, H.; Ravanshad, Y.; Zirachi, D.; Sahebari, M. Skin inflammatory reactions to capsaicin in rheumatoid arthritis patients compared to healthy controls. Avicenna J. Phytomed. 2019, 9, 54–61.
- Anand, P.; Bley, K. Topical capsaicin for pain management: Therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8 patch. Br. J. Anaesth. 2011, 107, 490–502.
- Yang, X.Y.; Du, G.H. Capsaicin. In Natural Small Molecule Drugs from Plants; Academic Press: Cambridge, MA, USA; Springer: Berlin/Heidelberg, Germany, 2018; pp. 397–402.
- Van Gerven, L.; Alpizar, Y.A.; Wouters, M.M.; Hox, V.; Hauben, E.; Jorissen, M.; Boeckxstaens, G.; Talavera, K.; Hellings, P.W. Capsaicin treatment reduces nasal hyperreactivity and transient receptor potential cation channel subfamily V, receptor 1 (TRPV1) overexpression in patients with idiopathic rhinitis. J. Allergy Clin. Immunol. 2014, 133, 1332–1339.
- Van Rijswijk, J.B.; Boeke, E.L.; Keizer, J.M.; Mulder, P.G.H.; Blom, H.M.; Fokkens, W.J. Intranasal capsaicin reduces nasal hyperreactivity in idiopathic rhinitis: A double-blind randomized application regimen study. Allergy Eur. J. Allergy Clin. Immunol. 2003, 58, 754–761.
This entry is offline, you can click here to edit this entry!
