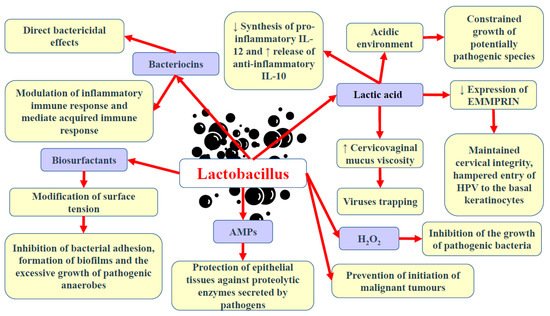
In contrast to many parts of the body in which great microbial diversity appears to be beneficial, in the vagina, a higher diversity of microbiota frequently results in dysbiosis and the development of disease states. Many studies have demonstrated that vaginal microbiota, including
Lactobacillus, is involved in the protection of the reproductive tract and gastrointestinal tract against opportunistic infections [
1,
7]. The ability of
Lactobacillus to produce lactic acid via the fermentation of glucose (glycolysis) supports vaginal eubiosis, as this organic acid helps preserve the vaginal acidic environment [
76]. The acidic environment constrains the growth of some potentially pathogenic species, including
C. trachomatis, G. vaginalis, and
Neisseria gonorrhoeae [
32,
77,
78,
79]. Vaginal pH exceeding 5.0 was found to increase the risk of HPV in premenopausal women by 10–20% [
80]. This finding could be partly explained by the fact that the HPV protein crucial for viral transformation, E5, is vulnerable to low pH [
81]. Moreover, it offers optimal conditions for the metabolic functioning of cervical and vaginal cells [
82]. Apart from affecting the pH of the environment, the chemical structure of lactic acid itself may modulate the HPV infection and the development of squamous intraepithelial lesions [
3]. As a chiral molecule, lactic acid can be produced in the form of D- and L-isomers. Studies demonstrated that high levels of D-lactic acid could protect against
Chlamydia infection and upper reproductive tract infections via the modulation of extracellular matrix metalloproteinase inducer (EMMPRIN) production in vaginal epithelial cells [
83,
84]. A higher L-lactate-to-D-lactate ratio is associated with the enhanced expression of EMMPRIN as well as the activation of matrix metalloproteinase 8 (MMP-8), eventually resulting in impaired cervical integrity and the easier entry of HPV into basal keratinocytes [
83]. Nunn et al. [
85] revealed that the predominance of
L. crispatus and relatively high levels of D-lactic acid could increase the viscosity of cervicovaginal mucus, resulting in viral particle trapping. Lactic acid also limits the cytotoxicity of natural killer (NK) cells, diminishes the synthesis of pro-inflammatory cytokine IL-12, and promotes the release of anti-inflammatory interleukin-10 (IL-10) [
86,
87]. Apart from lactic acid, beneficial microbiota can also release other antimicrobial peptides, including bacteriocins and hydrogen peroxide (H
2O
2) [
88,
89]. Bacteriocins exert direct bactericidal effects, but they can also modulate the inflammatory immune response and mediate acquired immune response [
1]. They possess anti-tumour properties resulting from cytotoxicity and the stimulation of cell lysis. Gassericin (bacteriocin), produced by
L. gasseri as well as other strains of
L. crispatus and
Lactobacillus reuteri, acts on Gram-negative and Gram-positive bacteria [
90,
91]. Apart from bacteriocins, some bacteria (e.g.,
Lactobacillus) can also release biosurfactants, which modify surface tension, therefore hampering bacterial adhesion, biofilm formation, and the excessive growth of pathogenic anaerobes [
92].
Lactobacillus epithelium adhesin (LEA), produced by
L. crispatus, prevents the pilus-mediated adhesion of
G. vaginalis [
93]. The aforementioned bacteriocins and biosurfactants have also been demonstrated to disturb viral infiltration [
94]. Moreover, both bacteriocin and surface-active components can constrain the synthesis of tumourigenic substances [
95]. A higher rate of bacterial vaginosis was reported in females with decreased vaginal levels of bacteria capable of producing H
2O
2 [
96]. The release of a variety of antimicrobial peptides (AMPs) into the uterine cavity poses a vital defence mechanism, protecting epithelial tissues against proteolytic enzymes secreted by pathogens [
97,
98]. Some studies have suggested that hypoxia could also promote the development of bacterial vaginosis since, in such conditions, bacteria are not able to produce H
2O
2 in a sufficient amount to inhibit pathogenic bacteria growth [
99,
100]. The interaction of commensal bacteria with endometrial epithelial cells was found to form an antimicrobial barrier against pathogens [
101]. The presence of
Lactobacillus in the vagina is associated with protection against the adherence of pathogenic bacteria to the epithelial tissue. These bacteria compete against pathogenic microorganisms for territories and nutrients [
102].
Lactobacillus that occupies the vaginal epithelial cells (VECs) has been found to prevent the conglutination of invasive pathogenic bacteria, thus hampering the initiation of malignant tumours [
103,
104].
Lactobacillus was demonstrated to hinder the proliferation of malignant tumours via the secretion of phosphorylated polysaccharides, exopolysaccharides, and peptidoglycans [
87,
105]. Moreover, these bacteria can stimulate nitric oxide (NO) production by macrophages and impair energy metabolism in cancer cells [
106]. Commensal bacteria stimulate the production of neutral, stable mucous by endometrial cells as well as preserve tight junctions [
65,
107]. An intact epithelial barrier is crucial for protection against the penetration and colonisation of opportunistic microorganisms. Furthermore, commensal bacteria can modify immune responses at the cellular level [
101]. Studies have demonstrated that
Lactobacillus enhances the proliferation and differentiation of thymus-derived cells (T cells) and ameliorates the immunological recognition and proliferation of B cells [
108,
109]. The adhesion of
Lactobacillus and the absorption of nutrients have been demonstrated to trigger the complement system, which subsequently regulates microbial growth [
110].
Motevaseli et al. [
111] demonstrated that vaginal lactobacilli (
L. gasseri and
L. crispatus) could exert cytotoxic impact on cervical tumour cells, however, normal cells remained unaffected. Moreover, they observed that this effect was independent of lactic acid and pH. Studies have demonstrated the antimetastatic and antiproliferative properties of
Lactobacillus, its subgenera, and its supernatants [
87]. Via the modulation of HPV oncogenes,
Lactobacillus was shown to limit cervical cancer cell viability. Another study has implied that
L. crispatus is highly resistant to the co-colonisation of other bacteria and the transition into CST IV [
46]. These bacteria are rarely found to coexist with other species. Furthermore, females with these bacteria have the lowest vaginal pH and are not susceptible to infections with bacterial STIs, HPV, herpes simplex virus-2 (HSV-2), or HIV [
31,
112]. Since bacterial vaginosis promotes the shedding of HIV and HSV-2, it has been suggested that dysbiosis and the reduced abundance of
Lactobacillus may support the formation of an environment that induces the persistence of infections and leads to the development of squamous intraepithelial lesions [
113]. The basic beneficial effects of Lactobacillus in the lower female genital tract are presented in
Figure 1.