Inflammatory bowel disease (IBD) is a chronic inflammatory disease comprising two major clinical entities—Crohn’s disease (CD) and ulcerative colitis (UC). IBD incidence remains constantly high in industrialized countries and continuously rises in emerging economies. Importantly, IBD is associated with neuropsychiatric symptoms that strongly worsen IBD disease burden. Mounting evidence indicates that chronic gut inflammation induces a systemic immune response that might cause the CNS manifestation in IBD. In line with this, biologicals targeting inflammatory circuits exerted robust positive effects on depressive symptoms in many autoimmune diseases, and in IBD in particular. Therefore, research in recent years increasingly focused on the characterization of local and systemic immune reactions in IBD, and on entry routes of inflammatory cells and molecules into the CNS. The ultimate aim is to understand how the changes in the neuroimmune landscape impair the function of neurons to cause neuropsychiatric symptoms. In addition, the role of intestinal microbiota in the gut–immune–brain axis in IBD will be discussed.
- inflammatory bowel disease
- neuroinflammation
- gut microbiota
- Crohn's disease
- ulcerative colitis
- systemic inflammation
- depression
- gut-brain axis
1. Routes from Peripheral Inflammation to the CNS in IBD
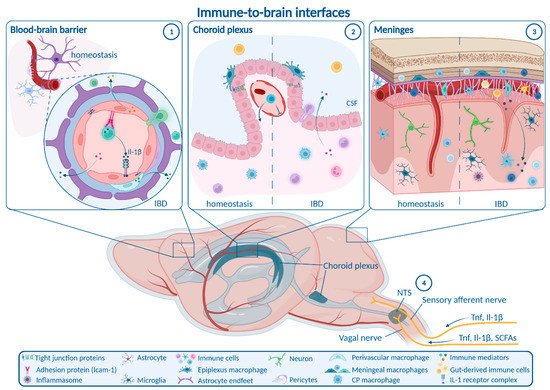
1.1. Enteric, Autonomic and Sensory Nervous System Signaling
1.2. Blood–Brain Barrier
1.3. Choroid Plexus and Blood–CSF Barrier
1.4. Meninges
2. How Neuroinflammation Is Linked to Depression and Anxiety
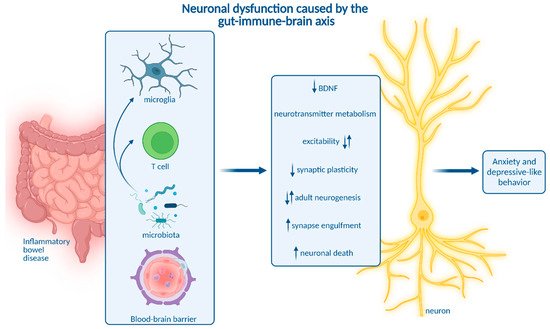
3. Impact of Microbiota on Neuroinflammation and Neuropsychiatric Disease
Moreover, intestinal microbiota are involved in neurotransmitter metabolism. Expansion of Bacteroides species contributes to depressive-like behavior and impaired hippocampal neurogenesis by regulating tryptophan and neurotransmitter metabolism [94]. Interestingly, Bacteroides species were also linked to the development of colitis [95]. Apart from that, gut microbiota are a major source of the neurotransmitter serotonin. Reduced serotonin abundance as a potential pathogenic mechanism driving depression may be caused by microbial dysbiosis and impaired serotonin production in the gastrointestinal tract [96].
In addition, gut microbiota influence the expression of micro-RNA (miRNA) in the gut and in different brain regions, which is associated with depression and anxiety in mice [97][98]. Interestingly, differential miRNA expression associated with microbiota dysbiosis distinguishes IBD patients and healthy individuals. MiRNAs are thus suggested as biomarkers and promising targets to treat intestinal inflammation [160].
This entry is adapted from the peer-reviewed paper 10.3390/ijms231911111
References
- Sigrid Breit; Aleksandra Kupferberg; Gerhard Rogler; Gregor Hasler; Vagus Nerve as Modulator of the Brain–Gut Axis in Psychiatric and Inflammatory Disorders. Frontiers in Psychiatry 2018, 9, 44, 10.3389/fpsyt.2018.00044.
- Takako Ichiki; Tongtong Wang; Ann Kennedy; Allan-Hermann Pool; Haruka Ebisu; David J. Anderson; Yuki Oka; Sensory representation and detection mechanisms of gut osmolality change. Nature 2022, 602, 468-474, 10.1038/s41586-021-04359-5.
- Chayon Goswami; Yusaku Iwasaki; Toshihiko Yada; Short-chain fatty acids suppress food intake by activating vagal afferent neurons. The Journal of Nutritional Biochemistry 2018, 57, 130-135, 10.1016/j.jnutbio.2018.03.009.
- Kirsteen N. Browning; Simon Verheijden; Guy E. Boeckxstaens; The Vagus Nerve in Appetite Regulation, Mood, and Intestinal Inflammation. Gastroenterology 2017, 152, 730-744, 10.1053/j.gastro.2016.10.046.
- Benjamin E. Steinberg; Harold A. Silverman; Sergio Robbiati; Manoj K. Gunasekaran; Téa Tsaava; Emily Battinelli; Andrew Stiegler; Chad E. Bouton; Sangeeta S. Chavan; Kevin J. Tracey; et al. Cytokine-specific Neurograms in the Sensory Vagus Nerve. Bioelectronic Medicine 2016, 3, 7-17, 10.15424/bioelectronmed.2016.00007.
- Linda R. Watkins; Lisa E. Goehler; Jane K. Relton; Nicole Tartaglia; Lee Silbert; David Martin; Steven F. Maier; Blockade of interleukin-1 induced hyperthermia by subdiaphragmatic vagotomy: evidence for vagal mediation of immune-brain communication. Neuroscience Letters 1995, 183, 27-31, 10.1016/0304-3940(94)11105-r.
- Premysl Bercik; Elena F. Verdu; Jane A. Foster; Joseph Macri; Murray Potter; Xiaxing Huang; Paul Malinowski; Wendy Jackson; Patricia Blennerhassett; Karen A. Neufeld; et al. Chronic Gastrointestinal Inflammation Induces Anxiety-Like Behavior and Alters Central Nervous System Biochemistry in Mice. Gastroenterology 2010, 139, 2102-2112.e1, 10.1053/j.gastro.2010.06.063.
- Peng Sun; Kewen Zhou; Sheng Wang; Ping Li; Sijuan Chen; Guiping Lin; Yan Zhao; Tinghuai Wang; Involvement of MAPK/NF-κB Signaling in the Activation of the Cholinergic Anti-Inflammatory Pathway in Experimental Colitis by Chronic Vagus Nerve Stimulation. PLOS ONE 2013, 8, e69424, 10.1371/journal.pone.0069424.
- Charles Ibeakanma; Stephen Vanner; TNFα is a key mediator of the pronociceptive effects of mucosal supernatant from human ulcerative colitis on colonic DRG neurons. Gut 2010, 59, 612-621, 10.1136/gut.2009.190439.
- Andreas Hess; Julie Roesch; Marc Saake; Marina Sergeeva; Simon Hirschmann; Helmut Neumann; Arnd Dörfler; Markus Friedrich Neurath; Raja Atreya; Functional Brain Imaging Reveals Rapid Blockade of Abdominal Pain Response Upon Anti-TNF Therapy in Crohn’s Disease. Gastroenterology 2015, 149, 864-866, 10.1053/j.gastro.2015.05.063.
- Britta Engelhardt; Peter Vajkoczy; Roy O Weller; The movers and shapers in immune privilege of the CNS. Nature Immunology 2017, 18, 123-131, 10.1038/ni.3666.
- Ian Galea; The blood–brain barrier in systemic infection and inflammation. Cellular & Molecular Immunology 2021, 18, 2489-2501, 10.1038/s41423-021-00757-x.
- Ilaria Spadoni; Elena Zagato; Alice Bertocchi; Roberta Paolinelli; Edina Hot; Antonio Di Sabatino; Flavio Caprioli; Luca Bottiglieri; Amanda Oldani; Giuseppe Viale; et al. A gut-vascular barrier controls the systemic dissemination of bacteria. Science 2015, 350, 830-834, 10.1126/science.aad0135.
- Aravinthan Varatharaj; Ian Galea; The blood-brain barrier in systemic inflammation. Brain, Behavior, and Immunity 2017, 60, 1-12, 10.1016/j.bbi.2016.03.010.
- Ying Han; Tong Zhao; Xiang Cheng; Ming Zhao; Sheng-Hui Gong; Yong-Qi Zhao; Hai-Tao Wu; Ming Fan; Ling-Ling Zhu; Cortical Inflammation is Increased in a DSS-Induced Colitis Mouse Model. Neuroscience Bulletin 2018, 34, 1058-1066, 10.1007/s12264-018-0288-5.
- Ying Han; Liping Ding; Xiang Cheng; Ming Zhao; Tong Zhao; Liang Guo; Xinyang Li; Yanan Geng; Ming Fan; Hong Liao; et al. Hypoxia Augments Cerebral Inflammation in a Dextran Sulfate Sodium-Induced Colitis Mouse Model. Frontiers in Cellular Neuroscience 2020, 14, 611764, 10.3389/fncel.2020.611764.
- Jonathon Mitchell; Su Jin Kim; Cody Howe; Seulah Lee; Ji Yun Her; Marisa Patel; Gayoung Kim; Jaewon Lee; Eunok Im; Sang Hoon Rhee; et al. Chronic Intestinal Inflammation Suppresses Brain Activity by Inducing Neuroinflammation in Mice. The American Journal of Pathology 2021, 192, 72-86, 10.1016/j.ajpath.2021.09.006.
- Sara Carloni; Alice Bertocchi; Sara Mancinelli; Martina Bellini; Marco Erreni; Antonella Borreca; Daniele Braga; Silvia Giugliano; Alessandro M. Mozzarelli; Daria Manganaro; et al. Identification of a choroid plexus vascular barrier closing during intestinal inflammation. Science 2021, 374, 439-448, 10.1126/science.abc6108.
- Xiaolin Zhao; Peng Liang; Jin Liu; Haixia Jiang; Xiaoshuai Fan; Guo Chen; Cheng Zhou; Elevation of arachidonoylethanolamide levels by activation of the endocannabinoid system protects against colitis and ameliorates remote organ lesions in mice. Experimental and Therapeutic Medicine 2017, 14, 5664-5670, 10.3892/etm.2017.5222.
- Christopher A. Hathaway; Caroline B. Appleyard; William H. Percy; John L. Williams; Experimental colitis increases blood-brain barrier permeability in rabbits. American Journal of Physiology-Gastrointestinal and Liver Physiology 1999, 276, G1174-G1180, 10.1152/ajpgi.1999.276.5.g1174.
- S. S. Natah; A. Mouihate; Q. J. Pittman; K. A. Sharkey; Disruption of the blood-brain barrier during TNBS colitis. Neurogastroenterology & Motility 2005, 17, 433-446, 10.1111/j.1365-2982.2005.00654.x.
- Sarah E. Barnes; Kristy A. Zera; Geoffrey T. Ivison; Marion S. Buckwalter; Edgar G. Engleman; Brain profiling in murine colitis and human epilepsy reveals neutrophils and TNFα as mediators of neuronal hyperexcitability. Journal of Neuroinflammation 2021, 18, 1-13, 10.1186/s12974-021-02262-4.
- Sarah Talley; Rasa Valiauga; Lillian Anderson; Abigail R. Cannon; Mashkoor A. Choudhry; Edward M. Campbell; DSS-induced inflammation in the colon drives a proinflammatory signature in the brain that is ameliorated by prophylactic treatment with the S100A9 inhibitor paquinimod. Journal of Neuroinflammation 2021, 18, 1-14, 10.1186/s12974-021-02317-6.
- Thomas Blank; Claudia N. Detje; Alena Spieß; Nora Hagemeyer; Stefanie M. Brendecke; Jakob Wolfart; Ori Staszewski; Tanja Zöller; Ismini Papageorgiou; Justus Schneider; et al. Brain Endothelial- and Epithelial-Specific Interferon Receptor Chain 1 Drives Virus-Induced Sickness Behavior and Cognitive Impairment. Immunity 2016, 44, 901-912, 10.1016/j.immuni.2016.04.005.
- Hanadie Yousef; Cathrin J. Czupalla; Davis Lee; Michelle B. Chen; Ashley Burke; Kristy Zera; Judith Zandstra; Elisabeth Berber; Benoit Lehallier; Vidhu Mathur; et al. Aged blood impairs hippocampal neural precursor activity and activates microglia via brain endothelial cell VCAM1. Nature Medicine 2019, 25, 988-1000, 10.1038/s41591-019-0440-4.
- Noha Althubaity; Julia Schubert; Daniel Martins; Tayyabah Yousaf; Maria A. Nettis; Valeria Mondelli; Carmine Pariante; Neil A. Harrison; Edward T. Bullmore; Danai Dima; et al. Choroid plexus enlargement is associated with neuroinflammation and reduction of blood brain barrier permeability in depression. NeuroImage: Clinical 2021, 33, 102926, 10.1016/j.nicl.2021.102926.
- Sriram Balusu; Elien Van Wonterghem; Riet De Rycke; Koen Raemdonck; Stephan Stremersch; Kris Gevaert; Marjana Brkic; Delphine Demeestere; Valerie Vanhooren; An Hendrix; et al. Identification of a novel mechanism of blood–brain communication during peripheral inflammation via choroid plexus‐derived extracellular vesicles. EMBO Molecular Medicine 2016, 8, 1162-1183, 10.15252/emmm.201606271.
- Hannah Van Hove; Liesbet Martens; Isabelle Scheyltjens; Karen De Vlaminck; Ana Rita Pombo Antunes; Sofie De Prijck; Niels Vandamme; Sebastiaan De Schepper; Gert Van Isterdael; Charlotte L. Scott; et al. A single-cell atlas of mouse brain macrophages reveals unique transcriptional identities shaped by ontogeny and tissue environment. Nature Neuroscience 2019, 22, 1021-1035, 10.1038/s41593-019-0393-4.
- Kuti Baruch; Aleksandra Deczkowska; Eyal David; Joseph M. Castellano; Omer Miller; Alexander Kertser; Tamara Berkutzki; Zohar Barnett-Itzhaki; Dana Bezalel; Tony Wyss-Coray; et al. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science 2014, 346, 89-93, 10.1126/science.1252945.
- Elaine Dempsey; Áine Abautret-Daly; Neil G. Docherty; Carlos Medina; Andrew Harkin; Persistent central inflammation and region specific cellular activation accompany depression- and anxiety-like behaviours during the resolution phase of experimental colitis. Brain, Behavior, and Immunity 2019, 80, 616-632, 10.1016/j.bbi.2019.05.007.
- Anthony J. Filiano; Yang Xu; Nicholas Tustison; Rachel L. Marsh; Wendy Baker; Igor Smirnov; Christopher C. Overall; Sachin P. Gadani; Stephen Turner; Zhiping Weng; et al. Unexpected role of interferon-γ in regulating neuronal connectivity and social behaviour. Nature 2016, 535, 425-429, 10.1038/nature18626.
- Kalil Alves De Lima; Justin Rustenhoven; Sandro Da Mesquita; Morgan Wall; Andrea Francesca Salvador; Igor Smirnov; Guilherme Martelossi Cebinelli; Tornike Mamuladze; Wendy Baker; Zach Papadopoulos; et al. Meningeal γδ T cells regulate anxiety-like behavior via IL-17a signaling in neurons. Nature Immunology 2020, 21, 1421-1429, 10.1038/s41590-020-0776-4.
- David Brea; Carrie Poon; Corinne Benakis; Gabrielle Lubitz; Michelle Murphy; Costantino Iadecola; Josef Anrather; Stroke affects intestinal immune cell trafficking to the central nervous system. Brain, Behavior, and Immunity 2021, 96, 295-302, 10.1016/j.bbi.2021.05.008.
- Pia Kivisäkk; Barbara Tucky; Tao Wei; James J Campbell; Richard M Ransohoff; Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: relevance for immunotherapy. BMC Immunology 2006, 7, 14-14, 10.1186/1471-2172-7-14.
- Xiao-Fei He; Li-Li Li; Wen-Biao Xian; Ming-Yue Li; Li-Ying Zhang; Jing-Hui Xu; Zhong Pei; Hai-Qing Zheng; Xi-Quan Hu; Chronic colitis exacerbates NLRP3-dependent neuroinflammation and cognitive impairment in middle-aged brain. Journal of Neuroinflammation 2021, 18, 153, 10.1186/s12974-021-02199-8.
- Ana Badimon; Hayley J. Strasburger; Pinar Ayata; Xinhong Chen; Aditya Nair; Ako Ikegami; Philip Hwang; Andrew T. Chan; Steven M. Graves; Joseph O. Uweru; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417-423, 10.1038/s41586-020-2777-8.
- Christopher N. Parkhurst; Guang Yang; Ipe Ninan; Jeffrey N. Savas; John R. Yates; Juan J. Lafaille; Barbara L. Hempstead; Dan R. Littman; Wen-Biao Gan; Microglia Promote Learning-Dependent Synapse Formation through Brain-Derived Neurotrophic Factor. Cell 2013, 155, 1596-1609, 10.1016/j.cell.2013.11.030.
- Emanuela Pasciuto; Oliver T. Burton; Carlos P. Roca; Vasiliki Lagou; Wenson D. Rajan; Tom Theys; Renzo Mancuso; Raul Y. Tito; Lubna Kouser; Zsuzsanna Callaerts-Vegh; et al. Microglia Require CD4 T Cells to Complete the Fetal-to-Adult Transition. Cell 2020, 182, 625-640.e24, 10.1016/j.cell.2020.06.026.
- Anna M. Klawonn; Michael Fritz; Silvia Castany; Marco Pignatelli; Carla Canal; Fredrik Similä; Hugo A. Tejeda; Julia Levinsson; Maarit Jaarola; Johan Jakobsson; et al. Microglial activation elicits a negative affective state through prostaglandin-mediated modulation of striatal neurons. Immunity 2021, 54, 225-234.e6, 10.1016/j.immuni.2020.12.016.
- Peng Cao; Changmao Chen; An Liu; Qinghong Shan; Xia Zhu; Chunhui Jia; Xiaoqi Peng; Mingjun Zhang; Zahra Farzinpour; Wenjie Zhou; et al. Early-life inflammation promotes depressive symptoms in adolescence via microglial engulfment of dendritic spines. Neuron 2021, 109, 2573-2589.e9, 10.1016/j.neuron.2021.06.012.
- Eric S. Wohleb; Rosemarie Terwilliger; Catharine H. Duman; Ronald S. Duman; Stress-Induced Neuronal Colony Stimulating Factor 1 Provokes Microglia-Mediated Neuronal Remodeling and Depressive-like Behavior. Biological Psychiatry 2017, 83, 38-49, 10.1016/j.biopsych.2017.05.026.
- Chenhui Ji; Yalin Tang; Yanyan Zhang; Congcong Li; Huazheng Liang; Lu Ding; Xiaohuan Xia; Lize Xiong; Xin-Rui Qi; Jialin C. Zheng; et al. Microglial glutaminase 1 deficiency mitigates neuroinflammation associated depression. Brain, Behavior, and Immunity 2021, 99, 231-245, 10.1016/j.bbi.2021.10.009.
- Weifen Li; Tahir Ali; Kaiwu He; Zizhen Liu; Fawad Ali Shah; Qingguo Ren; Yan Liu; Anlong Jiang; Shupeng Li; Ibrutinib alleviates LPS-induced neuroinflammation and synaptic defects in a mouse model of depression. Brain, Behavior, and Immunity 2020, 92, 10-24, 10.1016/j.bbi.2020.11.008.
- Fernanda N. Kaufmann; Ana Paula Costa; Gabriele Ghisleni; Alexandre Diaz; Ana Lúcia Rodrigues; Hugo Peluffo; Manuella P. Kaster; NLRP3 inflammasome-driven pathways in depression: Clinical and preclinical findings. Brain, Behavior, and Immunity 2017, 64, 367-383, 10.1016/j.bbi.2017.03.002.
- Ruozhi Dang; Mingyang Wang; Xinhui Li; Haiyang Wang; Lanxiang Liu; Qingyuan Wu; Jianting Zhao; Ping Ji; Lianmei Zhong; Julio Licinio; et al. Edaravone ameliorates depressive and anxiety-like behaviors via Sirt1/Nrf2/HO-1/Gpx4 pathway. Journal of Neuroinflammation 2022, 19, 1-29, 10.1186/s12974-022-02400-6.
- Tahir Ali; Qiang Hao; Najeeb Ullah; Shafiq Ur Rahman; Fawad Ali Shah; Kaiwu He; Chengyou Zheng; Weifen Li; Iram Murtaza; Yang Li; et al. Melatonin Act as an Antidepressant via Attenuation of Neuroinflammation by Targeting Sirt1/Nrf2/HO-1 Signaling. Frontiers in Molecular Neuroscience 2020, 13, 96, 10.3389/fnmol.2020.00096.
- Katarzyna A. Dudek; Laurence Dion-Albert; Manon Lebel; Katherine LeClair; Simon Labrecque; Ellen Tuck; Carmen Ferrer Perez; Sam A. Golden; Carol Tamminga; Gustavo Turecki; et al. Molecular adaptations of the blood–brain barrier promote stress resilience vs. depression. Proceedings of the National Academy of Sciences 2020, 117, 3326-3336, 10.1073/pnas.1914655117.
- Caroline Menard; Madeline L. Pfau; Georgia Hodes; Veronika Kana; Victoria X. Wang; Sylvain Bouchard; Aki Takahashi; Meghan E. Flanigan; Hossein Aleyasin; Katherine B. LeClair; et al. Social stress induces neurovascular pathology promoting depression. Nature Neuroscience 2017, 20, 1752-1760, 10.1038/s41593-017-0010-3.
- D B McKim; M D Weber; A Niraula; C M Sawicki; X Liu; B L Jarrett; K Ramirez-Chan; Y Wang; R M Roeth; A D Sucaldito; et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Molecular Psychiatry 2017, 23, 1421-1431, 10.1038/mp.2017.64.
- Zhi-Heng Zheng; Jiang-Long Tu; Xiao-Han Li; Qing Hua; Wei-Zhu Liu; Yu Liu; Bing-Xing Pan; Ping Hu; Wen-Hua Zhang; Neuroinflammation induces anxiety- and depressive-like behavior by modulating neuronal plasticity in the basolateral amygdala. Brain, Behavior, and Immunity 2020, 91, 505-518, 10.1016/j.bbi.2020.11.007.
- Kiarash Riazi; Michael A. Galic; J. Brent Kuzmiski; Winnie Ho; Keith A. Sharkey; Quentin J. Pittman; Microglial activation and TNFα production mediate altered CNS excitability following peripheral inflammation. Proceedings of the National Academy of Sciences 2008, 105, 17151-17156, 10.1073/pnas.0806682105.
- Colin F. Craig; Rhiannon T. Filippone; Rhian Stavely; Joel C. Bornstein; Vasso Apostolopoulos; Kulmira Nurgali; Neuroinflammation as an etiological trigger for depression comorbid with inflammatory bowel disease. Journal of Neuroinflammation 2022, 19, 1-30, 10.1186/s12974-021-02354-1.
- Jean-Eric Ghia; Nan Li; Huaqing Wang; Matthew Collins; Yikang Deng; Rami El-Sharkawy; Francine Côté; Jacques Mallet; Waliul I. Khan; Serotonin Has a Key Role in Pathogenesis of Experimental Colitis. Gastroenterology 2009, 137, 1649-1660, 10.1053/j.gastro.2009.08.041.
- Sharif Shajib; Adriana Baranov; Waliul Islam Khan; Diverse Effects of Gut-Derived Serotonin in Intestinal Inflammation. ACS Chemical Neuroscience 2017, 8, 920-931, 10.1021/acschemneuro.6b00414.
- Marie Skov Kristensen; Thora Majlund Kjærulff; Annette Kjær Ersbøll; Anders Green; Jesper Hallas; Lau Thygesen; The Influence of Antidepressants on the Disease Course Among Patients With Crohn’s Disease and Ulcerative Colitis—A Danish Nationwide Register–Based Cohort Study. Inflammatory Bowel Diseases 2018, 25, 886-893, 10.1093/ibd/izy367.
- Li-Ming Chen; Chun-Hui Bao; Yu Wu; Shi-Hua Liang; Di Di Wang; Lu-Yi Wu; Yan Huang; Hui-Rong Liu; Huan-Gan Wu; Tryptophan-kynurenine metabolism: a link between the gut and brain for depression in inflammatory bowel disease. Journal of Neuroinflammation 2021, 18, 1-13, 10.1186/s12974-021-02175-2.
- Hoda M. Sroor; Ahmed M. Hassan; Geraldine Zenz; Paulina Valadez-Cosmes; Aitak Farzi; Peter Holzer; Amany El-Sharif; Fatma Al-Zahraa M. Gomaa; Julia Kargl; Florian Reichmann; et al. Experimental colitis reduces microglial cell activation in the mouse brain without affecting microglial cell numbers. Scientific Reports 2019, 9, 1-12, 10.1038/s41598-019-56859-0.
- Patrick Süß; Microglia in Alzheimer’s Disease. Current Alzheimer Research 2020, 17, 29-43, 10.2174/1567205017666200212155234.
- Amanda Crider; Tami Feng; Chirayu D. Pandya; Talisha Davis; Ashwati Nair; Anthony O. Ahmed; Babak Baban; Gustavo Turecki; Anilkumar Pillai; Complement component 3a receptor deficiency attenuates chronic stress-induced monocyte infiltration and depressive-like behavior. Brain, Behavior, and Immunity 2018, 70, 246-256, 10.1016/j.bbi.2018.03.004.
- Jessica L. Bolton; Annabel K. Short; Shivashankar Othy; Cassandra L. Kooiker; Manlin Shao; Benjamin G. Gunn; Jaclyn Beck; Xinglong Bai; Stephanie M. Law; Julie C. Savage; et al. Early stress-induced impaired microglial pruning of excitatory synapses on immature CRH-expressing neurons provokes aberrant adult stress responses. Cell Reports 2022, 38, 110600, 10.1016/j.celrep.2022.110600.
- B L Jacobs; H van Praag; F H Gage; Adult brain neurogenesis and psychiatry: a novel theory of depression. Molecular Psychiatry 2000, 5, 262-269, 10.1038/sj.mp.4000712.
- Tomohisa Toda; Sarah L. Parylak; Sara B. Linker; Fred H. Gage; The role of adult hippocampal neurogenesis in brain health and disease. Molecular Psychiatry 2018, 24, 67-87, 10.1038/s41380-018-0036-2.
- Amanda Sierra; Juan Manuel Encinas; Juan José Peña Deudero; Jessica Chancey; Grigori Enikolopov; Linda Wadiche; Stella E. Tsirka; Mirjana Maletic-Savatic; Microglia Shape Adult Hippocampal Neurogenesis through Apoptosis-Coupled Phagocytosis. Cell Stem Cell 2010, 7, 483-495, 10.1016/j.stem.2010.08.014.
- Robert E. Iosif; Christine T. Ekdahl; Henrik Ahlenius; Cornelis J. H. Pronk; Sara Bonde; Zaal Kokaia; Sten Eirik Waelgaard Jacobsen; Olle Lindvall; Tumor Necrosis Factor Receptor 1 Is a Negative Regulator of Progenitor Proliferation in Adult Hippocampal Neurogenesis. The Journal of Neuroscience 2006, 26, 9703-9712, 10.1523/jneurosci.2723-06.2006.
- Inbal Goshen; T Kreisel; O Ben-Menachem-Zidon; Tamar Licht; J Weidenfeld; T Ben-Hur; R Yirmiya; Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular Psychiatry 2007, 13, 717-728, 10.1038/sj.mp.4002055.
- Michelle L. Monje; Hiroki Toda; Theo D. Palmer; Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science 2003, 302, 1760-1765, 10.1126/science.1088417.
- Maiko Tatsuki; Reiko Hatori; Tomoko Nakazawa; Takashi Ishige; Tomoko Hara; Seiichi Kagimoto; Takeshi Tomomasa; Hirokazu Arakawa; Takumi Takizawa; Serological cytokine signature in paediatric patients with inflammatory bowel disease impacts diagnosis. Scientific Reports 2020, 10, 1-11, 10.1038/s41598-020-71503-y.
- Saul A. Villeda; Jian Luo; Kira Mosher; Bende Zou; Markus Britschgi; Gregor Bieri; Trisha M. Stan; Nina Fainberg; Zhaoqing Ding; Alexander Eggel; et al. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature 2011, 477, 90-94, 10.1038/nature10357.
- Ioannis-Alexandros Gampierakis; Yassemi Koutmani; Maria Semitekolou; Ioannis Morianos; Alexia Polissidis; Antonia Katsouda; Ioannis Charalampopoulos; Georgina Xanthou; Achille Gravanis; Katia P. Karalis; et al. Hippocampal neural stem cells and microglia response to experimental inflammatory bowel disease (IBD). Molecular Psychiatry 2020, 26, 1248-1263, 10.1038/s41380-020-0651-6.
- Eloisa Salvo; Patricia Stokes; Ciara E. Keogh; Ingrid Brust-Mascher; Carly Hennessey; Trina A. Knotts; Jessica A. Sladek; Kavi M. Rude; Michelle Swedek; Gonzalo Rabasa; et al. A murine model of pediatric inflammatory bowel disease causes microbiota-gut-brain axis deficits in adulthood. American Journal of Physiology-Gastrointestinal and Liver Physiology 2020, 319, G361-G374, 10.1152/ajpgi.00177.2020.
- Svetlana Zonis; Robert N Pechnick; Vladimir A Ljubimov; Michael Mahgerefteh; Kolja Wawrowsky; Kathrin S Michelsen; Vera Chesnokova; Chronic intestinal inflammation alters hippocampal neurogenesis. Journal of Neuroinflammation 2015, 12, 1-12, 10.1186/s12974-015-0281-0.
- Osamu Nakagawasai; Kotaro Yamada; Kohei Takahashi; Takayo Odaira; Wakana Sakuma; Daisuke Ishizawa; Naruya Takahashi; Kentaro Onuma; Chikako Hozumi; Wataru Nemoto; et al. Liver hydrolysate prevents depressive-like behavior in an animal model of colitis: Involvement of hippocampal neurogenesis via the AMPK/BDNF pathway. Behavioural Brain Research 2020, 390, 112640, 10.1016/j.bbr.2020.112640.
- Kohei Takahashi; Kazuhiro Kurokawa; Kazuya Miyagawa; Atsumi Mochida-Saito; Yukio Nemoto; Hiroyuki Iwasa; Osamu Nakagawasai; Takeshi Tadano; Hiroshi Takeda; Minoru Tsuji; et al. Antidementia effects of Enterococcus faecalis 2001 are associated with enhancement of hippocampal neurogenesis via the ERK-CREB-BDNF pathway in olfactory bulbectomized mice. Physiology & Behavior 2020, 223, 112997, 10.1016/j.physbeh.2020.112997.
- Hyungju Park; Mu-Ming Poo; Neurotrophin regulation of neural circuit development and function. Nature Reviews Neuroscience 2012, 14, 7-23, 10.1038/nrn3379.
- Plinio C. Casarotto; Mykhailo Girych; Senem M. Fred; Vera Kovaleva; Rafael Moliner; Giray Enkavi; Caroline Biojone; Cecilia Cannarozzo; Madhusmita Pryiadrashini Sahu; Katja Kaurinkoski; et al. Antidepressant drugs act by directly binding to TRKB neurotrophin receptors. Cell 2021, 184, 1299-1313.e19, 10.1016/j.cell.2021.01.034.
- R.M Barrientos; D.B Sprunger; S Campeau; E.A Higgins; L.R Watkins; J.W Rudy; S.F Maier; Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 2003, 121, 847-853, 10.1016/s0306-4522(03)00564-5.
- Xiao Zhuang; Bing Zhan; Yufeng Jia; Chaoze Li; Nan Wu; Ming Zhao; Nuo Chen; Yaxin Guo; Yingxin Du; Yi Zhang; et al. IL-33 in the basolateral amygdala integrates neuroinflammation into anxiogenic circuits via modulating BDNF expression. Brain, Behavior, and Immunity 2022, 102, 98-109, 10.1016/j.bbi.2022.02.019.
- Arya Haj-Mirzaian; Shayan Amiri; Hossein Amini-Khoei; Mir-Jamal Hosseini; Arvin Haj-Mirzaian; Majid Momeny; Maryam Rahimi-Balaei; Ahmad Reza Dehpour; Anxiety- and Depressive-Like Behaviors are Associated with Altered Hippocampal Energy and Inflammatory Status in a Mouse Model of Crohn’s Disease. Neuroscience 2017, 366, 124-137, 10.1016/j.neuroscience.2017.10.023.
- Kiarash Riazi; Michael A. Galic; J. Brent Kuzmiski; Winnie Ho; Keith A. Sharkey; Quentin J. Pittman; Microglial activation and TNFα production mediate altered CNS excitability following peripheral inflammation. Proceedings of the National Academy of Sciences 2008, 105, 17151-17156, 10.1073/pnas.0806682105.
- Xiaomin Yuan; Biqing Chen; Zhenglan Duan; Ziqian Xia; Yang Ding; Tuo Chen; Huize Liu; Baosheng Wang; Bolin Yang; Xiaoyong Wang; et al. Depression and anxiety in patients with active ulcerative colitis: crosstalk of gut microbiota, metabolomics and proteomics. Gut Microbes 2021, 13, 1987779, 10.1080/19490976.2021.1987779.
- Fernando A. Vicentini; Jake C. Szamosi; Laura Rossi; Lateece Griffin; Kristoff Nieves; Dominique Bihan; Ian A. Lewis; Quentin J. Pittman; Mark G. Swain; Michael G. Surette; et al. Colitis-associated microbiota drives changes in behaviour in male mice in the absence of inflammation. Brain, Behavior, and Immunity 2022, 102, 266-278, 10.1016/j.bbi.2022.03.001.
- Mireia Valles-Colomer; Gwen Falony; Youssef Darzi; Ettje F. Tigchelaar; Jun Wang; Raul Y. Tito; Carmen Schiweck; Alexander Kurilshikov; Marie Joossens; Cisca Wijmenga; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nature Microbiology 2019, 4, 623-632, 10.1038/s41564-018-0337-x.
- Kohei Takahashi; Osamu Nakagawasai; Wataru Nemoto; Takayo Odaira; Wakana Sakuma; Hiroshi Onogi; Hiroaki Nishijima; Ryuji Furihata; Yukio Nemoto; Hiroyuki Iwasa; et al. Effect of Enterococcus faecalis 2001 on colitis and depressive-like behavior in dextran sulfate sodium-treated mice: involvement of the brain–gut axis. Journal of Neuroinflammation 2019, 16, 1-16, 10.1186/s12974-019-1580-7.
- Jacob R. Emge; Kevin Huynh; Elaine N. Miller; Manvir Kaur; Colin Reardon; Kim E. Barrett; Mélanie G. Gareau; Modulation of the microbiota-gut-brain axis by probiotics in a murine model of inflammatory bowel disease. American Journal of Physiology-Gastrointestinal and Liver Physiology 2016, 310, G989-G998, 10.1152/ajpgi.00086.2016.
- Roman Sankowski; Jasmin Ahmari; Charlotte Mezö; Anna Lena Hrabě de Angelis; Vidmante Fuchs; Olaf Utermöhlen; Thorsten Buch; Thomas Blank; Mercedes Gomez de Agüero; Andrew J Macpherson; et al. Commensal microbiota divergently affect myeloid subsets in the mammalian central nervous system during homeostasis and disease. The EMBO Journal 2021, 40, e108605, 10.15252/embj.2021108605.
- Alba Rodríguez-Nogales; Francesca Algieri; José Garrido-Mesa; Teresa Vezza; M. Pilar Utrilla; Natalia Chueca; Federico García; M. Elena Rodríguez-Cabezas; Julio Gálvez; Intestinal anti-inflammatory effect of the probiotic Saccharomyces boulardii in DSS-induced colitis in mice: Impact on microRNAs expression and gut microbiota composition. The Journal of Nutritional Biochemistry 2018, 61, 129-139, 10.1016/j.jnutbio.2018.08.005.
- Daniel Erny; Nikolaos Dokalis; Charlotte Mezö; Angela Castoldi; Omar Mossad; Ori Staszewski; Maximilian Frosch; Matteo Villa; Vidmante Fuchs; Arun Mayer; et al. Microbiota-derived acetate enables the metabolic fitness of the brain innate immune system during health and disease. Cell Metabolism 2021, 33, 2260-2276.e7, 10.1016/j.cmet.2021.10.010.
- Daniel Erny; Anna Lena Hrabě De Angelis; Diego Adhemar Jaitin; Peter Wieghofer; Ori Staszewski; Eyal David; Hadas Keren-Shaul; Tanel Mahlakoiv; Kristin Jakobshagen; Thorsten Buch; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nature Neuroscience 2015, 18, 965-977, 10.1038/nn.4030.
- Omar Mossad; Bérénice Batut; Bahtiyar Yilmaz; Nikolaos Dokalis; Charlotte Mezö; Elisa Nent; Lara Susann Nabavi; Melanie Mayer; Feres José Mocayar Maron; Joerg M. Buescher; et al. Gut microbiota drives age-related oxidative stress and mitochondrial damage in microglia via the metabolite N6-carboxymethyllysine. Nature Neuroscience 2022, 25, 295-305, 10.1038/s41593-022-01027-3.
- Alexander Duscha; Barbara Gisevius; Sarah Hirschberg; Nissan Yissachar; Gabriele I. Stangl; Eva Eilers; Verian Bader; Stefanie Haase; Johannes Kaisler; Christina David; et al. Propionic Acid Shapes the Multiple Sclerosis Disease Course by an Immunomodulatory Mechanism. Cell 2020, 180, 1067-1080.e16, 10.1016/j.cell.2020.02.035.
- Veit Rothhammer; Davis M. Borucki; Emily C. Tjon; Maisa C. Takenaka; Chun-Cheih Chao; Alberto Ardura-Fabregat; Kalil Alves de Lima; Cristina Gutiérrez-Vázquez; Patrick Hewson; Ori Staszewski; et al. Microglial control of astrocytes in response to microbial metabolites. Nature 2018, 557, 724-728, 10.1038/s41586-018-0119-x.
- Irina Leonardi; Iris H. Gao; Woan-Yu Lin; Megan Allen; Xin V. Li; William D. Fiers; Meghan Bialt De Celie; Gregory G. Putzel; Rhonda K. Yantiss; Melanie Johncilla; et al. Mucosal fungi promote gut barrier function and social behavior via Type 17 immunity. Cell 2022, 185, 831-846.e14, 10.1016/j.cell.2022.01.017.
- Miriam Bittel; Patrick Reichert; Ilann Sarfati; Anja Dressel; Stefanie Leikam; Stefan Uderhardt; Iris Stolzer; Tuan Anh Phu; Martin Ng; Ngan K. Vu; et al. Visualizing transfer of microbial biomolecules by outer membrane vesicles in microbe‐host‐communication in vivo. Journal of Extracellular Vesicles 2021, 10, e12159, 10.1002/jev2.12159.
- Youying Zhang; Qilin Fan; Yuanlong Hou; Xuanshuang Zhang; Zhe Yin; Xiaoying Cai; Wei Wei; Jiaying Wang; Dandan He; Guangji Wang; et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain, Behavior, and Immunity 2022, 102, 11-22, 10.1016/j.bbi.2022.02.007.
- Seth M. Bloom; Vinieth N. Bijanki; Gerardo M. Nava; Lulu Sun; Nicole P. Malvin; David L. Donermeyer; W. Michael Dunne; Paul M. Allen; Thaddeus S. Stappenbeck; Commensal Bacteroides Species Induce Colitis in Host-Genotype-Specific Fashion in a Mouse Model of Inflammatory Bowel Disease. Cell Host & Microbe 2011, 9, 390-403, 10.1016/j.chom.2011.04.009.
- Leon M. T. Dicks; Diron Hurn; Demi Hermanus; Gut Bacteria and Neuropsychiatric Disorders. Microorganisms 2021, 9, 2583, 10.3390/microorganisms9122583.
- Jian-Jun Chen; Ben-Hua Zeng; Wen-Wen Li; Chan-Juan Zhou; Song-Hua Fan; Ke Cheng; Li Zeng; Peng Zheng; Liang Fang; Hong Wei; et al. Effects of gut microbiota on the microRNA and mRNA expression in the hippocampus of mice. Behavioural Brain Research 2017, 322, 34-41, 10.1016/j.bbr.2017.01.021.
- A E Hoban; R M Stilling; Gerard Moloney; F Shanahan; T G Dinan; G Clarke; J F Cryan; The microbiome regulates amygdala-dependent fear recall. Molecular Psychiatry 2017, 23, 1134-1144, 10.1038/mp.2017.100.
- Coco Chu; Mitchell H. Murdock; Deqiang Jing; Tae Hyung Won; Hattie Chung; Adam Kressel; Tea Tsaava; Meghan E. Addorisio; Gregory G. Putzel; Lei Zhou; et al. The microbiota regulate neuronal function and fear extinction learning. Nature 2019, 574, 543-548, 10.1038/s41586-019-1644-y.
- Omar Mossad; Elisa Nent; Sabrina Woltemate; Shani Folschweiller; Joerg M. Buescher; Daniel Schnepf; Daniel Erny; Peter Staeheli; Marlene Bartos; Antal Szalay; et al. Microbiota-dependent increase in δ-valerobetaine alters neuronal function and is responsible for age-related cognitive decline. Nature Aging 2021, 1, 1127-1136, 10.1038/s43587-021-00141-4.
- Fernando A. Vicentini; Catherine M. Keenan; Laurie E. Wallace; Crystal Woods; Jean-Baptiste Cavin; Amanda R. Flockton; Wendy B. Macklin; Jaime Belkind-Gerson; Simon A. Hirota; Keith A. Sharkey; et al. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome 2021, 9, 1-24, 10.1186/s40168-021-01165-z.
