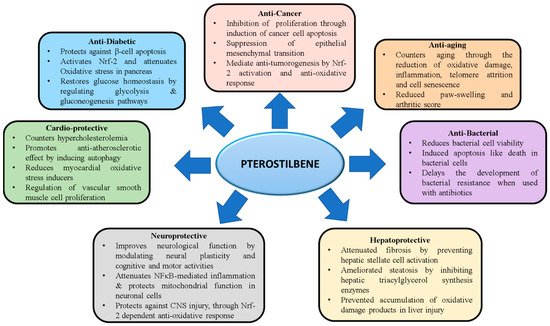
Pterostilbene (PTS), a natural analog of resveratrol is a compound most abundantly found in blueberries. PTS is produced by several plant species such as peanuts and grapes. While resveratrol has been extensively studied for its antioxidant properties, recent evidence also points out the diverse therapeutic potential of PTS. Several studies have identified the robust pharmacodynamic features of PTS, including better intestinal absorption and elevated hepatic stability than resveratrol. Indeed, due to its higher bioavailability paired with reduced toxicity compared to other stilbenes, PTS has become an attractive drug candidate for the treatment of several disease conditions, including diabetes, cancer, cardiovascular disease, neurodegenerative disorders, COVID-19 and aging.
- pterostilbene
- resveratrol
- antioxidant
- bioavailability
- cancer
- diabetes
Pterostilbene
Therapeutic Properties of PTS
Anti-Cancer Activity of PTS
Anti-Diabetic Activity of PTS
Therapeutic Effect of PTS in Liver Diseases
Effects of PTS on Diseases of the Central Nervous System
Effects of PTS on Cardiovascular Diseases
Effects of PTS on Aging
Antibacterial Effect of PTS
Potential Therapeutic Effects of PTS against COVID-19 Infection
Conclusion and future prospects
Several studies that have evaluated PTS for its therapeutic potential have demonstrated its role as a promising candidate drug for health benefits in a broad spectrum of disease conditions. Various experimental studies have confirmed that PTS has anticancer, anti-diabetic, anti-hypertensive, antimicrobial, anti-aging, anti-atherosclerotic, and neuroprotective properties. PTS exerted its beneficial effects mainly by modulating antioxidant, anti-apoptotic, and anti-inflammatory pathways. Even at higher doses, PTS did not exhibit toxicity in animal studies, providing further encouragement to explore the use of the compound in more human clinical trials. However, considering that some of the studies have employed co-administration of PTS in combination with other compounds to improve its therapeutic efficiency, the potential effect of drug interactions should be considered. Although PTS has been identified to exert marked therapeutic benefits, most findings have been proven only in experimental models. Human clinical trials have been largely limited due to the lesser-than-desired bioavailability of the compound. To overcome this limitation, various strategies have been implemented, which involve modifying the administration routes and formulations of PTS ranging from co-crystals, pro-drugs, nanoparticles, lipid-based encapsulation, and beads. The potential effect of many of drug interactions with PTS still remains unclear. Of note, the mode of administration of PTS seems to play an important role in its bioavailability, as the administration of intravenous doses show a higher distribution when compared to oral intake. Further research that carefully considers the dose, drug interactions, administration route, disease-specific formulations, and the short and long-term biomedical implications are warranted before clinical adoption of this promising natural compound.
This entry is adapted from the peer-reviewed paper 10.3390/molecules27196316
References
- Wang, P.; Sang, S. Metabolism and Pharmacokinetics of Resveratrol and Pterostilbene. Biofactors 2018, 44, 16–25.
- Estrela, J.M.; Ortega, A.; Mena, S.; Rodriguez, M.L.; Asensi, M. Pterostilbene: Biomedical Applications. Crit. Rev. Clin. Lab. Sci. 2013, 50, 65–78.
- Kosuru, R.; Rai, U.; Prakash, S.; Singh, A.; Singh, S. Promising Therapeutic Potential of Pterostilbene and Its Mechanistic Insight Based on Preclinical Evidence. Eur. J. Pharmacol. 2016, 789, 229–243.
- Kapetanovic, I.M.; Muzzio, M.; Huang, Z.; Thompson, T.N.; McCormick, D.L. Pharmacokinetics, Oral Bioavailability, and Metabolic Profile of Resveratrol and Its Dimethylether Analog, Pterostilbene, in Rats. Cancer Chemother. Pharmacol. 2011, 68, 593–601.
- Langcake, P.; Cornford, C.A.; Pryce, R.J. Identification of Pterostilbene as a Phytoalexin from Vitis Vinifera Leaves. Phytochemistry 1979, 18, 1025–1027.
- Rodríguez-Bonilla, P.; López-Nicolás, J.M.; Méndez-Cazorla, L.; García-Carmona, F. Development of a Reversed Phase High Performance Liquid Chromatography Method Based on the Use of Cyclodextrins as Mobile Phase Additives to Determine Pterostilbene in Blueberries. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2011, 879, 1091–1097.
- McCormack, D.; McFadden, D. A Review of Pterostilbene Antioxidant Activity and Disease Modification. Oxid. Med. Cell Longev. 2013, 2013, 575482.
- Remsberg, C.M.; Yáñez, J.A.; Ohgami, Y.; Vega-Villa, K.R.; Rimando, A.M.; Davies, N.M. Pharmacometrics of Pterostilbene: Preclinical Pharmacokinetics and Metabolism, Anticancer, Antiinflammatory, Antioxidant and Analgesic Activity. Phytother. Res. 2008, 22, 169–179.
- Chan, E.W.C.; Wong, C.W.; Tan, Y.H.; Foo, J.P.Y.; Wong, S.K.; Chan, H.T. Resveratrol and Pterostilbene: A Comparative Overview of Their Chemistry, Biosynthesis, Plant Sources and Pharmacological Properties. J. App. Pharm. Sci. 2019, 9, 124–129.
- Chen, R.-J.; Kuo, H.-C.; Cheng, L.-H.; Lee, Y.-H.; Chang, W.-T.; Wang, B.-J.; Wang, Y.-J.; Cheng, H.-C. Apoptotic and Nonapoptotic Activities of Pterostilbene against Cancer. Int. J. Mol. Sci. 2018, 19, 287.
- Rimando, A.; Cuendet, M.; Desmarchelier, C.; Mehta, R.; Pezzuto, J.; Duke, S. Cancer Chemopreventive and Antioxidant Activities of Pterostilbene, a Naturally Occurring Analogue of Resveratrol. J. Agric. Food Chem. 2002, 50, 3453–3457.
- Elsherbini, A.M.; Sheweita, S.A.; Sultan, A.S. Pterostilbene as a Phytochemical Compound Induces Signaling Pathways Involved in the Apoptosis and Death of Mutant P53-Breast Cancer Cell Lines. Nutr. Cancer 2021, 73, 1976–1984.
- Su, C.-M.; Lee, W.-H.; Wu, A.T.H.; Lin, Y.-K.; Wang, L.-S.; Wu, C.-H.; Yeh, C.-T. Pterostilbene Inhibits Triple-Negative Breast Cancer Metastasis via Inducing MicroRNA-205 Expression and Negatively Modulates Epithelial-to-Mesenchymal Transition. J. Nutr. Biochem. 2015, 26, 675–685.
- Tam, K.-W.; Ho, C.-T.; Tu, S.-H.; Lee, W.-J.; Huang, C.-S.; Chen, C.-S.; Wu, C.-H.; Lee, C.-H.; Ho, Y.-S. α-Tocopherol Succinate Enhances Pterostilbene Anti-Tumor Activity in Human Breast Cancer Cells in Vivo and in Vitro. Oncotarget 2017, 9, 4593–4606.
- Mannal, P.; McDonald, D.; McFadden, D. Pterostilbene and Tamoxifen Show an Additive Effect against Breast Cancer in Vitro. Am. J. Surg. 2010, 200, 577–580.
- Priego, S.; Feddi, F.; Ferrer, P.; Mena, S.; Benlloch, M.; Ortega, A.; Carretero, J.; Obrador, E.; Asensi, M.; Estrela, J.M. Natural Polyphenols Facilitate Elimination of HT-29 Colorectal Cancer Xenografts by Chemoradiotherapy: A Bcl-2- and Superoxide Dismutase 2-Dependent Mechanism. Mol. Cancer Ther. 2008, 7, 3330–3342.
- Paul, S.; DeCastro, A.J.; Lee, H.J.; Smolarek, A.K.; So, J.Y.; Simi, B.; Wang, C.X.; Zhou, R.; Rimando, A.M.; Suh, N. Dietary Intake of Pterostilbene, a Constituent of Blueberries, Inhibits the β-Catenin/P65 Downstream Signaling Pathway and Colon Carcinogenesis in Rats. Carcinogenesis 2010, 31, 1272–1278.
- Chiou, Y.-S.; Tsai, M.-L.; Nagabhushanam, K.; Wang, Y.-J.; Wu, C.-H.; Ho, C.-T.; Pan, M.-H. Pterostilbene Is More Potent than Resveratrol in Preventing Azoxymethane (AOM)-Induced Colon Tumorigenesis via Activation of the NF-E2-Related Factor 2 (Nrf2)-Mediated Antioxidant Signaling Pathway. J. Agric. Food Chem. 2011, 59, 2725–2733.
- Ferrer, P.; Asensi, M.; Segarra, R.; Ortega, A.; Benlloch, M.; Obrador, E.; Varea, M.T.; Asensio, G.; Jordá, L.; Estrela, J.M. Association between Pterostilbene and Quercetin Inhibits Metastatic Activity of B16 Melanoma. Neoplasia 2005, 7, 37–47.
- Mena, S.; Rodríguez, M.L.; Ponsoda, X.; Estrela, J.M.; Jäättela, M.; Ortega, A.L. Pterostilbene-Induced Tumor Cytotoxicity: A Lysosomal Membrane Permeabilization-Dependent Mechanism. PLoS ONE 2012, 7, e44524.
- Tsai, M.-L.; Lai, C.-S.; Chang, Y.-H.; Chen, W.-J.; Ho, C.-T.; Pan, M.-H. Pterostilbene, a Natural Analogue of Resveratrol, Potently Inhibits 7,12-DimethylbenzAnthracene (DMBA)/12-O-Tetradecanoylphorbol-13-Acetate (TPA)-Induced Mouse Skin Carcinogenesis. Food Funct. 2012, 3, 1185–1194.
- Sirerol, J.A.; Feddi, F.; Mena, S.; Rodriguez, M.L.; Sirera, P.; Aupí, M.; Pérez, S.; Asensi, M.; Ortega, A.; Estrela, J.M. Topical Treatment with Pterostilbene, a Natural Phytoalexin, Effectively Protects Hairless Mice against UVB Radiation-Induced Skin Damage and Carcinogenesis. Free Radic. Biol. Med. 2015, 85, 1–11.
- Obrador, E.; Salvador-Palmer, R.; Jihad-Jebbar, A.; López-Blanch, R.; Dellinger, T.H.; Dellinger, R.W.; Estrela, J.M. Pterostilbene in Cancer Therapy. Antioxidants 2021, 10, 492.
- Benlloch, M.; Obrador, E.; Valles, S.L.; Rodriguez, M.L.; Sirerol, J.A.; Alcácer, J.; Pellicer, J.A.; Salvador, R.; Cerdá, C.; Sáez, G.T.; et al. Pterostilbene Decreases the Antioxidant Defenses of Aggressive Cancer Cells In Vivo: A Physiological Glucocorticoids- and Nrf2-Dependent Mechanism. Antioxid. Redox. Signal. 2016, 24, 974–990.
- Kong, Y.; Chen, G.; Xu, Z.; Yang, G.; Li, B.; Wu, X.; Xiao, W.; Xie, B.; Hu, L.; Sun, X.; et al. Pterostilbene Induces Apoptosis and Cell Cycle Arrest in Diffuse Large B-Cell Lymphoma Cells. Sci. Rep. 2016, 6, 37417.
- Xie, B.; Xu, Z.; Hu, L.; Chen, G.; Wei, R.; Yang, G.; Li, B.; Chang, G.; Sun, X.; Wu, H.; et al. Pterostilbene Inhibits Human Multiple Myeloma Cells via ERK1/2 and JNK Pathway In Vitro and In Vivo. Int. J. Mol. Sci. 2016, 17, 1927.
- Wang, D.; Guo, H.; Yang, H.; Wang, D.; Gao, P.; Wei, W. Pterostilbene, An Active Constituent of Blueberries, Suppresses Proliferation Potential of Human Cholangiocarcinoma via Enhancing the Autophagic Flux. Front. Pharmacol. 2019, 10, 1238.
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene, a Natural Phenolic Compound, Synergizes the Antineoplastic Effects of Megestrol Acetate in Endometrial Cancer. Sci. Rep. 2017, 7, 12754.
- Wen, W.; Lowe, G.; Roberts, C.M.; Finlay, J.; Han, E.S.; Glackin, C.A.; Dellinger, T.H. Pterostilbene Suppresses Ovarian Cancer Growth via Induction of Apoptosis and Blockade of Cell Cycle Progression Involving Inhibition of the STAT3 Pathway. Int. J. Mol. Sci. 2018, 19, 1983.
- Guo, L.; Tan, K.; Wang, H.; Zhang, X. Pterostilbene Inhibits Hepatocellular Carcinoma through P53/SOD2/ROS-Mediated Mitochondrial Apoptosis. Oncol. Rep. 2016, 36, 3233–3240.
- Wang, R.; Xu, Z.; Tian, J.; Liu, Q.; Dong, J.; Guo, L.; Hai, B.; Liu, X.; Yao, H.; Chen, Z.; et al. Pterostilbene Inhibits Hepatocellular Carcinoma Proliferation and HBV Replication by Targeting Ribonucleotide Reductase M2 Protein. Am. J. Cancer Res. 2021, 11, 2975–2989.
- Qian, Y.-Y.; Liu, Z.-S.; Yan, H.-J.; Yuan, Y.-F.; Levenson, A.S.; Li, K. Pterostilbene Inhibits MTA1/HDAC1 Complex Leading to PTEN Acetylation in Hepatocellular Carcinoma. Biomed. Pharmacother. 2018, 101, 852–859.
- Baquer, N.Z.; Gupta, D.; Raju, J. Regulation of Metabolic Pathways in Liver and Kidney during Experimental Diabetes: Effects of Antidiabetic Compounds. Indian J. Clin. Biochem. 1998, 13, 63–80.
- Satheesh, M.A.; Pari, L. The Antioxidant Role of Pterostilbene in Streptozotocin-Nicotinamide-Induced Type 2 Diabetes Mellitus in Wistar Rats. J. Pharm. Pharmacol. 2010, 58, 1483–1490.
- Bhakkiyalakshmi, E.; Sireesh, D.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Anti-Hyperlipidemic and Anti-Peroxidative Role of Pterostilbene via Nrf2 Signaling in Experimental Diabetes. Eur. J. Pharmacol. 2016, 777, 9–16.
- Pari, L.; Satheesh, M.A. Effect of Pterostilbene on Hepatic Key Enzymes of Glucose Metabolism in Streptozotocin- and Nicotinamide-Induced Diabetic Rats. Life Sci. 2006, 79, 641–645.
- Sireesh, D.; Ganesh, M.-R.; Dhamodharan, U.; Sakthivadivel, M.; Sivasubramanian, S.; Gunasekaran, P.; Ramkumar, K.M. Role of Pterostilbene in Attenuating Immune Mediated Devastation of Pancreatic Beta Cells via Nrf2 Signaling Cascade. J. Nutr. Biochem. 2017, 44, 11–21.
- Dornadula, S.; Thiruppathi, S.; Palanisamy, R.; Umapathy, D.; Suzuki, T.; Mohanram, R.K. Differential Proteomic Profiling Identifies Novel Molecular Targets of Pterostilbene against Experimental Diabetes. J. Cell. Physiol. 2019, 234, 1996–2012.
- Acharya, J.D.; Ghaskadbi, S.S. Protective Effect of Pterostilbene against Free Radical Mediated Oxidative Damage. BMC Complement. Altern. Med. 2013, 13, 238.
- Zhang, Y.; Ren, S.; Ji, Y.; Liang, Y. Pterostilbene Ameliorates Nephropathy Injury in Streptozotocin-Induced Diabetic Rats. Pharmacology 2019, 104, 71–80.
- Shen, H.; Rong, H. Pterostilbene Impact on Retinal Endothelial Cells under High Glucose Environment. Int. J. Clin. Exp. Pathol. 2015, 8, 12589–12594.
- Schuppan, D.; Afdhal, N.H. Liver Cirrhosis. Lancet 2008, 371, 838–851.
- Lee, M.-F.; Liu, M.-L.; Cheng, A.-C.; Tsai, M.-L.; Ho, C.-T.; Liou, W.-S.; Pan, M.-H. Pterostilbene Inhibits Dimethylnitrosamine-Induced Liver Fibrosis in Rats. Food Chem. 2013, 138, 802–807.
- Aguirre, L.; Palacios-Ortega, S.; Fernández-Quintela, A.; Hijona, E.; Bujanda, L.; Portillo, M.P. Pterostilbene Reduces Liver Steatosis and Modifies Hepatic Fatty Acid Profile in Obese Rats. Nutrients 2019, 11, 961.
- Tsai, H.-Y.; Shih, Y.-Y.; Yeh, Y.-T.; Huang, C.-H.; Liao, C.-A.; Hu, C.-Y.; Nagabhushanam, K.; Ho, C.-T.; Chen, Y.-K. Pterostilbene and Its Derivative 3’-Hydroxypterostilbene Ameliorated Nonalcoholic Fatty Liver Disease Through Synergistic Modulation of the Gut Microbiota and SIRT1/AMPK Signaling Pathway. J. Agric. Food Chem. 2022, 70, 4966–4980.
- Zhang, H.; Chen, Y.; Chen, Y.; Ji, S.; Jia, P.; Xu, J.; Li, Y.; Wang, T. Pterostilbene Attenuates Liver Injury and Oxidative Stress in Intrauterine Growth–Retarded Weanling Piglets. Nutrition 2021, 81, 110940.
- Kim, H.; Seo, K.-H.; Yokoyama, W. Chemistry of Pterostilbene and Its Metabolic Effects. J. Agric. Food Chem. 2020, 68, 12836–12841.
- Joseph, J.A.; Fisher, D.R.; Cheng, V.; Rimando, A.M.; Shukitt-Hale, B. Cellular and Behavioral Effects of Stilbene Resveratrol Analogues: Implications for Reducing the Deleterious Effects of Aging. J. Agric. Food Chem. 2008, 56, 10544–10551.
- Chang, J.; Rimando, A.; Pallas, M.; Camins, A.; Porquet, D.; Reeves, J.; Shukitt-Hale, B.; Smith, M.A.; Joseph, J.A.; Casadesus, G. Low-Dose Pterostilbene, but Not Resveratrol, Is a Potent Neuromodulator in Aging and Alzheimer’s Disease. Neurobiol. Aging 2012, 33, 2062–2071.
- Yang, Y.; Wang, J.; Li, Y.; Fan, C.; Jiang, S.; Zhao, L.; Di, S.; Xin, Z.; Wang, B.; Wu, G.; et al. HO-1 Signaling Activation by Pterostilbene Treatment Attenuates Mitochondrial Oxidative Damage Induced by Cerebral Ischemia Reperfusion Injury. Mol. Neurobiol. 2015, 53, 2339–2353.
- Song, Z.; Han, S.; Pan, X.; Gong, Y.; Wang, M. Pterostilbene Mediates Neuroprotection against Oxidative Toxicity via Oestrogen Receptor α Signalling Pathways. J. Pharm. Pharmacol. 2015, 67, 720–730.
- Yang, Y.; Fan, C.; Wang, B.; Ma, Z.; Wang, D.; Gong, B.; Di, S.; Jiang, S.; Li, Y.; Li, T.; et al. Pterostilbene Attenuates High Glucose-Induced Oxidative Injury in Hippocampal Neuronal Cells by Activating Nuclear Factor Erythroid 2-Related Factor 2. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 827–837.
- Poulose, S.M.; Thangthaeng, N.; Miller, M.G.; Shukitt-Hale, B. Effects of Pterostilbene and Resveratrol on Brain and Behavior. Neurochem. Int. 2015, 89, 227–233.
- Sinha, K.; Chaudhary, G.; Kumar Gupta, Y. Protective Effect of Resveratrol against Oxidative Stress in Middle Cerebral Artery Occlusion Model of Stroke in Rats. Life Sci. 2002, 71, 655–665.
- Zhang, F.; Shi, J.-S.; Zhou, H.; Wilson, B.; Hong, J.-S.; Gao, H.-M. Resveratrol Protects Dopamine Neurons against Lipopolysaccharide-Induced Neurotoxicity through Its Anti-Inflammatory Actions. Mol. Pharmacol. 2010, 78, 466–477.
- Lacerda, D.; Türck, P.; Campos-Carraro, C.; Hickmann, A.; Ortiz, V.; Bianchi, S.; Belló-Klein, A.; de Castro, A.L.; Bassani, V.L.; da Rosa Araujo, A.S. Pterostilbene Improves Cardiac Function in a Rat Model of Right Heart Failure through Modulation of Calcium Handling Proteins and Oxidative Stress. Appl. Physiol. Nutr. Metab. 2020, 45, 987–995.
- Kosuru, R.; Kandula, V.; Rai, U.; Prakash, S.; Xia, Z.; Singh, S. Pterostilbene Decreases Cardiac Oxidative Stress and Inflammation via Activation of AMPK/Nrf2/HO-1 Pathway in Fructose-Fed Diabetic Rats. Cardiovasc. Drugs Ther. 2018, 32, 147–163.
- Mechanisms of atherosclerosis--a review. Adv. Nephrol. Necker Hosp. 1990, 19, 79–86.
- Park, E.-S.; Lim, Y.; Hong, J.-T.; Yoo, H.-S.; Lee, C.-K.; Pyo, M.-Y.; Yun, Y.-P. Pterostilbene, a Natural Dimethylated Analog of Resveratrol, Inhibits Rat Aortic Vascular Smooth Muscle Cell Proliferation by Blocking Akt-Dependent Pathway. Vasc. Pharmacol. 2010, 53, 61–67.
- Li, Y.-R.; Li, S.; Lin, C.-C. Effect of Resveratrol and Pterostilbene on Aging and Longevity. Biofactors 2018, 44, 69–82.
- Hou, Y.; Xie, G.; Miao, F.; Ding, L.; Mou, Y.; Wang, L.; Su, G.; Chen, G.; Yang, J.; Wu, C. Pterostilbene Attenuates Lipopolysaccharide-Induced Learning and Memory Impairment Possibly via Inhibiting Microglia Activation and Protecting Neuronal Injury in Mice. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2014, 54, 92–102.
- Porquet, D.; Casadesús, G.; Bayod, S.; Vicente, A.; Canudas, A.M.; Vilaplana, J.; Pelegrí, C.; Sanfeliu, C.; Camins, A.; Pallàs, M.; et al. Dietary Resveratrol Prevents Alzheimer’s Markers and Increases Life Span in SAMP8. AGE 2013, 35, 1851–1865.
- Li, J.; Deng, R.; Hua, X.; Zhang, L.; Lu, F.; Coursey, T.G.; Pflugfelder, S.C.; Li, D.-Q. Blueberry Component Pterostilbene Protects Corneal Epithelial Cells from Inflammation via Anti-Oxidative Pathway. Sci. Rep. 2016, 6, 19408.
- Wilson, M.A.; Shukitt-Hale, B.; Kalt, W.; Ingram, D.K.; Joseph, J.A.; Wolkow, C.A. Blueberry Polyphenols Increase Lifespan and Thermotolerance in Caenorhabditis Elegans. Aging Cell 2006, 5, 59–68.
- Peng, C.; Zuo, Y.; Kwan, K.M.; Liang, Y.; Ma, K.Y.; Chan, H.Y.E.; Huang, Y.; Yu, H.; Chen, Z.-Y. Blueberry Extract Prolongs Lifespan of Drosophila Melanogaster. Exp. Gerontol. 2012, 47, 170–178.
- Joseph, J.A.; Denisova, N.A.; Arendash, G.; Gordon, M.; Diamond, D.; Shukitt-Hale, B.; Morgan, D. Blueberry Supplementation Enhances Signaling and Prevents Behavioral Deficits in an Alzheimer Disease Model. Nutr. Neurosci. 2003, 6, 153–162.
- Majeed, M.; Majeed, S.; Jain, R.; Mundkur, L.; Rajalakshmi, H.; Lad, P.S.; Neupane, P. An Open-Label Single-Arm, Monocentric Study Assessing the Efficacy and Safety of Natural Pterostilbene (Pterocarpus Marsupium) for Skin Brightening and Antiaging Effects. Clin. Cosmet. Investig. Dermatol. 2020, 13, 105–116.
- Rui, Z.; Zhang, L.; Li, X.; Han, J.; Yuan, Y.; Ding, H.; Liu, Y.; Ding, X. Pterostilbene Exert an Anti-Arthritic Effect by Attenuating Inflammation, Oxidative Stress, and Alteration of Gut Microbiota. J. Food. Biochem. 2022, 46, e14011.
- Vaňková, E.; Paldrychová, M.; Kašparová, P.; Lokočová, K.; Kodeš, Z.; Maťátková, O.; Kolouchová, I.; Masák, J. Natural Antioxidant Pterostilbene as an Effective Antibiofilm Agent, Particularly for Gram-Positive Cocci. World J. Microbiol. Biotechnol. 2020, 36, 101.
- Lim, Y.R.I.; Preshaw, P.M.; Lim, L.P.; Ong, M.M.A.; Lin, H.-S.; Tan, K.S. Pterostilbene Complexed with Cyclodextrin Exerts Antimicrobial and Anti-Inflammatory Effects. Sci. Rep. 2020, 10, 9072.
- Shih, Y.-H.; Tsai, P.-J.; Chen, Y.-L.; Pranata, R.; Chen, R.-J. Assessment of the Antibacterial Mechanism of Pterostilbene against Bacillus Cereus through Apoptosis-like Cell Death and Evaluation of Its Beneficial Effects on the Gut Microbiota. J. Agric. Food Chem. 2021, 69, 12219–12229.
- Lee, W.X.; Basri, D.F.; Ghazali, A.R. Bactericidal Effect of Pterostilbene Alone and in Combination with Gentamicin against Human Pathogenic Bacteria. Molecules 2017, 22, 463.
- Yang, S.-C.; Tseng, C.-H.; Wang, P.-W.; Lu, P.-L.; Weng, Y.-H.; Yen, F.-L.; Fang, J.-Y. Pterostilbene, a Methoxylated Resveratrol Derivative, Efficiently Eradicates Planktonic, Biofilm, and Intracellular MRSA by Topical Application. Front. Microbiol. 2017, 8, 1103.
- ter Ellen, B.M.; Dinesh Kumar, N.; Bouma, E.M.; Troost, B.; van de Pol, D.P.I.; van der Ende-Metselaar, H.H.; Apperloo, L.; van Gosliga, D.; van den Berge, M.; Nawijn, M.C.; et al. Resveratrol and Pterostilbene Inhibit SARS-CoV-2 Replication in Air–Liquid Interface Cultured Human Primary Bronchial Epithelial Cells. Viruses 2021, 13, 1335.
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective Inhibition of MERS-CoV Infection by Resveratrol. BMC Infect. Dis. 2017, 17, 144.
- Das, A.; Pandita, D.; Jain, G.K.; Agarwal, P.; Grewal, A.S.; Khar, R.K.; Lather, V. Role of Phytoconstituents in the Management of COVID-19. Chem. Biol. Interact. 2021, 341, 109449.
- Kelleni, M.T. Resveratrol-Zinc Nanoparticles or Pterostilbene-Zinc: Potential COVID-19 Mono and Adjuvant Therapy. Biomed Pharmacother. 2021, 139, 111626.
- Hsieh, Y.-H.; Chen, C.W.S.; Schmitz, S.-F.H.; King, C.-C.; Chen, W.-J.; Wu, Y.-C.; Ho, M.-S. Candidate Genes Associated with Susceptibility for SARS-Coronavirus. Bull. Math. Biol. 2010, 72, 122–132.
- Hu, W.; Yu, H.; Zhou, X.; Li, M.; Xiao, L.; Ruan, Q.; Huang, X.; Li, L.; Xie, W.; Guo, X.; et al. Topical Administration of Pterostilbene Accelerates Burn Wound Healing in Diabetes through Activation of the HIF1α Signaling Pathway. Burns 2021, 48, 1452–1461.
