The atrial natriuretic peptide (ANP), a cardiovascular hormone, plays a pivotal role in the homeostatic control of blood pressure, electrolytes, and water balance and is approved to treat congestive heart failure. In addition, there is a growing realization that ANPs might be related to immune response and tumor growth. The anti-inflammatory and immune-modulatory effects of ANPs in the tissue microenvironment are mediated through autocrine or paracrine mechanisms, which further suppress tumorigenesis. In cancers, ANPs show anti-proliferative effects through several molecular pathways. Furthermore, ANPs attenuate the side effects of cancer therapy. Therefore, ANPs act on several hallmarks of cancer, such as inflammation, angiogenesis, sustained tumor growth, and metastasis.
1. Modulation of Inflammation and Anti-Tumor Effects
Innate and adaptive immunity either promotes or inhibits various aspects of tumor development, as well as regulates responses to anti-cancer therapy [
67]. The relationship between the immune system and carcinogenesis, as well as response to anti-tumor therapy, is intimate. The documenting of leukocytes within tumors, as observed in the 19th century by Rudolf Virchow, first linked cancer with inflammation [
68]. This hypothesis gained clear evidence hereafter, and tumor-associated inflammation has been recognized as a hallmark of cancer [
69]. Chronic inflammation drives tumorigenesis and promotes tumor progression and metastasis, as well as drug resistance [
70].
ANPs can counteract the occurrence of inflammation and inflammasome activation in the TME to inhibit tumor development. Persistent inflammation is a potent risk factor for neoplastic transformation [
71]. ANPs were shown to inhibit LPS-induced pro-inflammatory enzyme expression, NF-κB activation, and pro-inflammatory cytokine secretion [
24]. Meanwhile, NPRA-disrupted mice have shown higher expressions of pro-inflammatory cytokines, such as TNF-α, IL-6, and TGF-β1 [
72,
73]. Subramanian et al. reported that 4 weeks of ANP treatment significantly inhibited skin cancer development in a mouse model of skin cancer. Significant reductions in the NF-κB activation levels, mast cell infiltration numbers, and MMP-2/9 levels in the skin tissues of mice treated with ANPs were observed, while the changes in serum lactate dehydrogenase-4, C-reactive protein, and enzymatic antioxidants (superoxide dismutase and catalase activities) were close to normal [
74]. Chronic inflammation promotes prostate cancer progress and drug resistance [
75]. Prostate cancer cells secrete extracellular vesicles (EVs) loaded with pro-inflammatory molecules to modify the tumor microenvironment and promote tumor progression [
76]. Mezzasoma et al. reported that ANPs phosphorylated the NLRP3 receptor through the p38-MAPK pathway and then inhibited the inflammasome activation and IL-β maturation in PC3 cells, as well as reversing the inflammatory phenotypes of normal cells induced by EVs from PC3 cells [
77].
ANPs are a potent agent to inhibit perioperative systemic inflammation and postoperative cancer recurrence. If the primary solid tumor meets surgical indications and the patient’s physical condition permits, the surgical removal of tumors remains a mainstay attempt to cure patients. However, surgical trauma not only provokes the detachment of cancer cells, leading to an increase in circulating tumor cell (CTC) count, but it also causes a severe systemic inflammatory reaction that speeds up the adhesion of CTCs to the endothelium of distant organs [
78,
79,
80,
81]. This is an important step in hematogenous metastases. Nojiri et al. reported that, in a large-scale observational clinical study, the perioperative administration of low-dose human ANPs reduced inflammatory responses and postoperative cardiopulmonary complications in patients receiving surgical treatment for lung cancer [
82,
83]. Interestingly, patients treated with ANPs had longer 2-year relapse-free survival times [
84]. In the lungs of tumor-bearing mice, a significant inactivation of the ANP–NPRA pathway was noticed at the pre-metastatic niche, and ANP treatment downregulated pre-metastatic niche factors, thus preventing lung metastasis [
84]. Therefore, the ANP–NPRA pathway may have potential therapeutic value in preventing the pre-metastatic niche formation of solid cancers.
NPRA signaling provides a critical link between inflammation and tumorigenesis. ANPs exert biological effects through interacting with two specific plasma membrane receptors: NPRA and NPRC. Kong et al. reported that the NH (2)-terminal peptide of the ANP prohormone NP73-102 exerted robust anti-inflammatory and anti-tumor effects by blocking the expression of NPRA [
85]. In addition, in an endothelial-sprouting assay, an NPRA antagonist reduced NPRA expression and inhibited inflammation-induced angiogenesis by downregulating vascular endothelial growth factor (VEGF) and chemokine (C-X-C motif) receptor 4 (CXCR4). The accumulation of cancer-associated fibroblasts, endothelial cells, and macrophages decreased in an NPRA-disrupted mouse tumor microenvironment compared to WT mice, suggesting that NPRA signaling promoted tumor–stroma interaction. In addition, the absence of NPRA caused mesenchymal stem cells (MSCs) to fail to migrate to the TME. In contrast, significant increases in angiogenesis and tumorigenesis were noticed in NPRA-disrupted mice when co-implanting tumor cells with MSCs. These findings suggest that CXCR4 expression and stromal-derived factor 1α secretion are dependent on NPRA signaling [
86]. Thus, NPRA signaling may be a potential therapeutic target for inflammation-associated tumorigenesis.
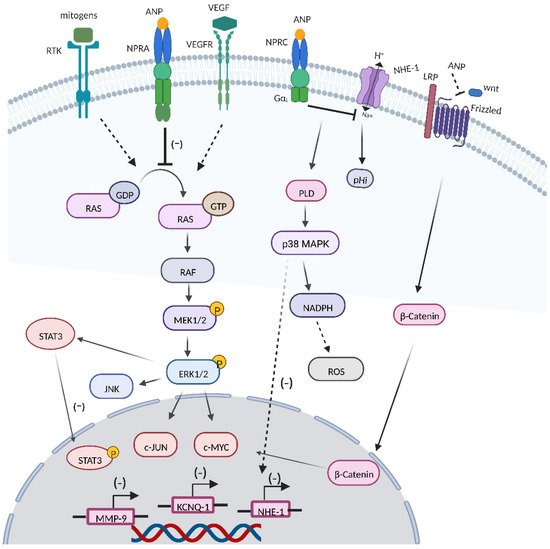
2. RAS-MEK1/2-ERK1/2 Kinase Cascade
Oncogenic mutated forms of Ras are detected in approximately 15% of cancers, and ERK hyperactivation can be seen in nearly one-third of human cancers, leading to deregulation of the RAS-mitogen-activated protein kinase (MEK)-extracellular-signal-regulated kinase (ERK) signaling pathway [
87]. Sun et al. reported that ANP and LANP treatment could effectively inhibit the conversion of the RAS-GDP signal to the RAS-GTP signal in prostate cancer cells [
88]. In addition, ANPs could suppress the activation of MEK1/2 and ERK1/2 in prostate cancer cells, and the inhibitory effect could be largely abolished by the cGMP antibody [
89]. EGF and insulin were shown to stimulate ERK 1/2 kinases through mediating conversion of RAS-GDP to active RAS-GTP [
90]. What is more, ANPs could block the activation of RAS and ERK 1/2 by mitogens such as insulin and epidermal growth factor (EGF) [
91].
3. ANPs Interact with Other Transcription Factors and Cell Signaling Systems
3.1. VEGF
Tumor cells and the surrounding stroma can secrete VEGF [
92]. The overexpression of VEGF is related to tumor vascular density, invasiveness, metastasis, recurrence, and prognosis, and the blockading of VEGF may lead to a regression in the vascular network and the containment of tumor growth [
93,
94]. Given the fact that vascular endothelium cells express high levels of NPRA and that cell exposure to ANPs can counteract VEGF-induced endothelial cell proliferation signals, the ANP/NPR1 signal plays a critical role in the regulation of endothelial cell functional activity and proliferation [
35]. Levin et al. showed that ANP inhibited the activation of several key signaling molecules, including ERK, JNK, and p38 members of the MAP kinase family, that were important for VEGF-induced angiogenesis [
20]. ANPs could significantly block VEGF-induced endothelial cell proliferation and migration [
95]. Nguyen et al. reported that ANPs could inhibit VEGF and VEGF receptor 2 in human cancer cell lines [
96]. In addition, Mao et al. showed that ANPs combined with glipizide significantly suppressed breast cancer growth by inhibiting tumor-induced angiogenesis [
97]. Moreover, Nakao et al. showed that high NPRA expression in tongue squamous cell carcinoma had a poorer prognosis, and NPRA was related to the expressions of VEGFA and VEGFC, which were associated with the invasion potential of tongue squamous cell carcinoma [
98].
3.2. Wnt/β-Catenin Signaling Cascade
The dysregulation of the Wnt/β-catenin signal caused by mutations or epigenetic changes contributes to the initiation and development of various human cancers [
99]. ANPs downregulated β-catenin expression and caused a significant downregulation of c-Myc and cyclin D-1 transcriptions [
65]. An acidic microenvironment has been identified as critical factor for the development of cancer. ANPs can modify the pH by inhibiting or stimulating NHE-1 [
100,
101]. Serafino et al. showed that ANPs induced NHE-1 inactivation, leading to an increase in intracellular acidity, inhibiting Wnt/β-catenin signaling through a frizzled-mediated activation that was on the upstream of the cascade, simultaneously [
26].
3.3. JNK and JAK/STATs Signaling
The c-JUN N-terminal kinase (JNK) pathway is involved in a range of pathological conditions, including inflammation and cancer progression [
102]. ANPs could reduce the expression of JNK2 in human prostate adenocarcinoma and small-cell lung cancer cells [
28]. The signal transducer and activator of transcription (STAT3) signal is constitutively activated during cancer development and is associated with different hallmarks of cancer [
103]. Lane et al. showed that ANPs inhibited the expression of STAT3 in human cancer cells [
65].
3.4. ROS Production
Cancer cells show higher level of ROS than normal cells, partly due to high metabolic activity, peroxisomal activity, and mitochondrial dysfunction, which contributes to tumorigenesis, tumor progression, and metastasis, as well as drug resistance [
104,
105,
106]. When the levels of ROS fluctuate, cancer cells more easily reach their oxidative stress thresholds compare to normal cells, resulting in oxidative-stress-induced cell death [
107,
108]. In hepG2 cells, increased expression of ANP-inhibited cell growth was seen through the upregulation of NPRC [
64]. Baldini et al. showed that, in ANP-treated HepG2 cells, a significant decrease in pHi, enhancement in the PLD activity, and an increase in the ROS level were observed simultaneously [
109]. Furthermore, ANP-induced ROS generation via the involvement of the NADPH oxidase system subsequently inhibited the caspase-3 enzyme and switched HepG2 cell death from apoptosis to necrosis [
110]. However, Kiemers et al. showed that ANP had a cytoprotective effect on liver cells after ischemia reperfusion injury [
111], which might be attributed to the differences between tumor cells and normal cells in terms of threshold signal, NPR-C expression, and intracellular ROS level [
23].
3.5. KCNQ1 Expression
Plasma K
+ channels are important in tumor cell proliferation [
112,
113]. Zhang et al. showed that ANPs played a pleiotropic role in gastric cancer cell proliferation, and the physiological concentration of ANPs upregulated the expression of the potassium-voltage-gated channel and KQT-like subfamily member 1 (KCNQ1) to promote AGS cell proliferation, while the pharmacological concentration of ANP significantly downregulated KCNQ1 expression to inhibit AGS proliferation [
114]. Furthermore, the knockdown of NPRA decreased the protein level of KCNQ1 and the voltage-gated outward K
+ current [
115].
3.6. MMP9 Expression
The dysregulation of MMP9 is related to several hallmarks of cancer, including inflammation, angiogenesis, tumor growth, and metastasis [
116]. Li et al. reported that ANPs significantly inhibited gastric cancer cell migration and invasion by downregulating the hedgehog-signaling-mediated activation of MMP9 [
117]. In vivo, ANP treatment significantly reduced NF-κB activation levels, mast cell infiltration numbers, and MMP2/9 levels in the skin tissues of a skin cancer mouse model [
74] (
Figure 4).
Figure 4. Roles of ANPs in cancer. ANPs exert antineoplastic potential by inhibiting the conversion of GDP-RAS to GTP-RAS, the RAS-MEK1/2-ERK1/2 kinase cascade, and cross-talk between the RAS-MEK1/2-ERK1/2 kinase cascade and VEGF, β-catenin, JNK, WNT, and STAT3. In addition, ANPs also modulate inflammation, ROS production, KCNQ1, and MMP9 expression.
This entry is adapted from the peer-reviewed paper 10.3390/cancers14163981