Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Modified vaccinia virus Ankara (MVA) is a promising viral vector for vaccine development. MVA is well studied and has been widely used for vaccination against smallpox in Germany.
- viral vector vaccines
- poxvirus
- smallpox vaccine
- vaccine development
1. Introduction
In recent years, viral vectors have become widely used in the development of new vaccines. This is due, first, to the high immunogenicity of these vectors, which mimic a natural infection and thus effectively stimulate two main arms of the adaptive immune response, as well as cell-mediated immunity. Another important factor is the high safety of viral vector vaccines compared to live attenuated vaccines. The most widely used viral vectors are derived from adenovirus, vaccinia virus, measles virus, herpes virus, and vesicular stomatitis virus. Among them, the vaccinia virus (VACV) is the only virus that was not originally a human pathogen. On the contrary, it became the first vaccine in human history directed against the smallpox virus. It is thanks to the massive worldwide vaccination with VACV that smallpox was eradicated. Studies of VACV led to the production of new strains, one of which, modified vaccinia virus Ankara (MVA), has been widely used as a vaccine vector since, along with the high immunogenicity typical of VACV, it had a high level of safety.
2. History of the Origin of the Vaccinia Strain MVA and Its Properties
Until the 1960s, strains of VACV, which varied greatly in their biological properties, were used in different countries for smallpox vaccination [1]. The first strains of VACV were named after the health care institution, country, or area of origin. The most widely used strains are shown in Table 1 which was adapted from Sanchez-Sampredo et al [2]. Detailed information about the history of smallpox and the origin of VACV can be found in the study by Kaynarcalidan et al. [3].
Table 1. List of the first VACV strains used for vaccination [2].
| VACV Strain | Country or Region of Application |
|---|---|
| New York City Board of Health (NYCBOH), Dryvax |
USA |
| Lister | UK, Europe, Asia, Africa, USA |
| Temple of Heaven (Tian Tan) | China |
| Tashkent, Gam, MRIVP, Per, B-51 | USSR |
| Lister/L-IVP | USSR/Russian Federation |
| Bern | Germany, Austria |
| Paris | France, Syria, Turkey |
| Copenhagen | Denmark |
| Dairen, Ikeda | Japan |
| Sweden | Sweden |
| Ankara | Turkey |
| Chambon | France and Africa |
For the preparation of the first-generation vaccine, a live virus produced on the skin of calves was used [4], so there was a high probability of microbial contamination of the preparations. In addition, such vaccines contained animal proteins, which often caused allergies in patients. Later, the cultivation of vaccine strains began to be carried out on the chorioallantoic membrane of chicken embryos or in various cell lines. This method led to the emergence of second-generation vaccines [2].
Numerous studies have shown that the use of cell cultures to grow viruses allowed for better control of vaccine production. This led to the stable production of the virus and an increase in the purity of the vaccine preparation due to the elimination of contamination by bacteria and animal proteins. However, the frequency of side effects that are characteristic of viral infections (e.g., fever, headache, malaise, and muscle aches) remained high with the second-generation vaccines. In addition, in rare cases, severe side effects such as post-vaccination encephalitis, myocarditis, and pericarditis, which required hospitalization and could lead to death, were observed [2,5,6]. Given the poor safety profile of the second-generation VACV vaccines, efforts have been made to improve their safety, leading to the third-generation vaccines [7].
Multiple passaging of the parental vaccine strain has been used to generate random mutations and deletions, which in turn have led to the attenuation of VACV and the emergence of new strains such as Lister-16m8 (LC16m8) [8], Dairen I (DIs) [9], M65 and M101 [10], NYVAC [11], ACAM3000 [4], and modified vaccinia virus Ankara (MVA) [12]. The origins of these strains and their genetic features are described in detail in the review by Kaynarcalidan et al. [3]. Of all the strains, only MVA and NYVAC are unable to replicate in human cells [13].
MVA was developed by Anton Mayr and Eberhard Munz in the 1960s as a result of sequential infection of primary chicken embryo fibroblasts (PEFs) with the chorioallantois VACV Ankara (CVA) vaccine strain, which was used for smallpox vaccination in Turkey and Germany [14]. After 516 passages, a highly attenuated laboratory virus was obtained and given the name MVA. The new virus differed from the parental CVA by the phenotype of the virus-infected chicken embryo cells. As a result of CVA infection, the fusion of squamous cells occurred, and continuous lysis was observed. In the case of MVA infection, infected cells took on a spherical shape, and the formation of individual plaques (limited areas with lysed cells) was observed. Most importantly, during the process of attenuation, MVA completely lost its ability to replicate in mammalian cells (with the exception of the Syrian hamster fibroblast cell line (BHK-21)) [2,15,16]. It is the inability to replicate in mammalian cells that distinguishes MVA from the other third-generation vaccinia strains; thus, MVA is currently considered one of the safest strains of the vaccinia virus.
Genomic studies have shown that the parental CVA strain lost approximately 15% of its genome [12], reducing the genome length from 208 kb in CVA to 177 kb in MVA [17]. Six large genomic deletions were identified, ranging in length from 2.6 kb to 10.2 kb, as well as many shorter deletions, insertions, and point mutations, leading to fragmentation, truncation, or deletion of open reading frames (ORFs) (Figure 1) [18]. As a result of these modifications, MVA ceased to encode many virulence factors, including factors that suppress the immune response to the vaccinia virus, such as viral receptors for γ-interferon, α/β interferons, and CC chemokines [19].
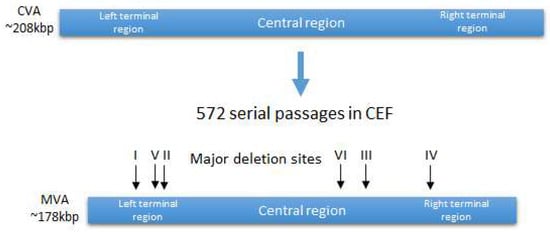
Figure 1. Generation of MVA strain from CVA strain. Location of the major deletion sites.
In 2019 [20] and 2020 [21], restoration of the C12L and C16L/B22R genes was identified as necessary to restore the ability of MVA to productively infect mammalian cells. The functions of these genes have not yet been studied enough, but it is known that they affect the synthesis and processing of the late structural proteins of the virus. In the absence of these genes in human and other mammalian cells, the virus replicates its DNA and has undisturbed expression of early and intermediate genes, as well as of most of the late genes, but further development is blocked at the stage of virion assembly [18], which makes it impossible to form infectious progeny [22,23,24,25].
3. Safety of Smallpox Vaccines Based on the MVA Strain
A smallpox vaccine based on the MVA strain was first used in Germany in 1977. Due to its high safety profile, it was used for children, the elderly, and people with weakened immune systems [1,26]. In just a few years, more than 120,000 people were vaccinated.
Vaccinated patients experienced mild local reactions, such as redness at the injection site, but did not develop blisters, pustules, or ulcers, as was observed with the second-generation smallpox vaccines [27,28,29,30]. In 2.3% of cases, vaccination caused a fever, and in 4.1% of cases, it led to the development of other non-specific systemic reactions [26], but no severe side effects were detected. Since vaccination was carried out at a time when smallpox was no longer present in Germany, its effectiveness remained unexplored [31].
Another smallpox vaccine based on the MVA strain, MVA-BN (V00083008), was licensed by the European Medicines Agency in 2013 under the brand name IMVAMUNE and by the US Food and Drug Administration (FDA) in 2019 under the brand name Jynneos [32]. According to several clinical studies in immunocompromised people, including those with atopic dermatitis or HIV infection, the MVA-BN vaccine has been shown to be completely safe and highly immunogenic [33,34,35,36,37]. The vaccine was recommended in the US and Canada in 2022 to prevent monkeypox [38]. These authorizations were based on the results obtained in the study by Earl et al. [39]. This work showed that two vaccinations with MVA-BN completely protect cynomolgus monkeys from a lethal monkeypox infection.
One of the most serious complications when using the first- and second-generation smallpox vaccines was neurotoxicity. The frequency of post-vaccination encephalitis varied depending on the vaccine strain. In the past, the heavily used Lister and Dryvax® vaccines averaged 2.6 and 2.9 cases of vaccinal encephalitis per million doses, respectively [40,41]. Although the incidence of encephalitis after vaccination was extremely low, a quarter of these cases ended in death, and in another quarter, irreversible neurological disorders developed [5,42,43,44]. No cases of neurotoxicity were recorded with third-generation LC16m8 or MVA vaccines [45]. Animal studies have also demonstrated that intracerebral inoculation of MVA does not lead to encephalitis, and, moreover, immunization with MVA prevents the risk of developing encephalitis after vaccination with a replication-competent vaccine [16,46,47].
The most commonly reported severe adverse events with the first- and second-generation smallpox vaccines were myocarditis and pericarditis, which occurred at a rate of 120 cases per million [48,49,50]. The MVA strain vaccine has not been shown to increase the risk of myo- or pericarditis [36,51,52].
Moreover, preliminary vaccination with MVA has been shown to be able to attenuate skin lesions caused by the first-generation Dryvax vaccine based on the replication-competent vaccinia strain NYCBOH.
It was further shown that the safety of the MVA virus depends on its homogeneity. MVA obtained by Mayr [15] has long been considered incapable of replication in mammalian cells [12,23]. However, in 2009, new data showed that at least some MVA strains deposited in different collections, such as MVA-572 (ECACC), MVA-I721 (National Collection of Cultures of Microorganisms, CNCM, Pasteur Institute Paris), MVA VR-1508 (ATCC), are heterogeneous and contain virus variants that can replicate in human cell lines and even cause lethality in immunodeficient mice [28,53,54]. At the same time, it was shown that the MVA-BN strain, obtained as a result of six rounds of purification of the MVA-584 strain by the selection of individual plaques (viral clones), was not able to replicate in any of the human cell lines considered in the study or in mice with suppressed immunity [53]. The obtained data indicate the need for a careful assessment of the homogeneity of those strains that are planned for use in clinical practice.
Furthermore, there were concerns about the possible restoration of MVA replication as a result of its recombination with circulating orthopoxviruses in vivo, for example, when vaccinating animals. This situation was simulated in vitro by infecting cells permissive to MVA with replication-competent Norwegian vaccinia strain No-H1 simultaneously with MVA [55]. A hybrid MVA that could propagate in human cells could only be obtained by infecting the cell line with high doses of both viruses (multiplicity of infection = 5), an extremely unlikely event in vivo. It should be noted that no occurrence of a replication-competent MVA strain has been reported in any preclinical or clinical study [56].
4. Recombinant Vaccines Based on MVA
In addition to being used as a smallpox vaccine, the MVA virus can also serve as a vector for vaccines against other pathogens. MVA is considered a promising viral vector due to its ability to incorporate up to 25 kb of foreign DNA into its genome, to express a wide range of transgenes with correct post-translational modification, due to its high immunogenicity in vivo [57,58], and also because of its safety profile.
To date, a large number of candidate vaccines have been developed based on the MVA vector, including vaccines against HIV [59,60], tuberculosis [61], malaria [62,63,64], Ebola [65,66,67,68,69], RSV [70,71], MERS [72], CMV [73], and influenza [74,75,76], which are being studied in the late stages of clinical trials. Significantly more candidate vaccines against a variety of other human diseases appear in preclinical studies [2,18,77]. MVA is also an attractive and efficient viral vector for the development of recombinant veterinary vaccines [78,79,80,81,82,83,84,85,86,87].
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10091516
This entry is offline, you can click here to edit this entry!
