Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Cell Biology
β-galactosidase is a lysosomal hydrolase, which cleaves terminal β-d-galactose residues. SA-β-gal assay found its place in the routine work of thousands of biological laboratories. There is a bulk of evidence that supports the future implementation of β-galactosidase detection as a prognostic marker in medical practice as well.
- cellular senescence
- senescence-associated beta-galactosidase
- oncology
1. Medical Applications of SA-β-Gal Assay
SA-β-gal assay found its place in the routine work of thousands of biological laboratories. There is a bulk of evidence that supports the future implementation of β-galactosidase detection as a prognostic marker in medical practice as well [13]. The expression of this enzyme in cells increases in many pathological conditions, especially in inflammation, various dystrophies, and tumors [14].
Oncopathology is the area in which studies of β-galactosidase activity are most numerous [15]. This enzyme can be detected both in benign formations and precancerous conditions and in malignant tumors. It is known that defects in proteins that regulate the cell cycle are common triggers causing the development of cancer. Rearrangement, amplification, and overexpression of cyclins D1, E, and A occur both in hematopoietic diseases and carcinomas of various organs. β-galactosidase can be detected in neoplasias of various organs, which puts it on par with such universal cell cycle-associated proteins as p53, bcl2, PTEN, ki-67. The enzyme is considered to be a product of a suppressor gene that provides prevention of tumor cell transformation [16]. Evaluation of SA-β-gal expression will give a significant impetus to the development of diagnostics of many tumors of various localizations and understanding of their morphogenesis, as well as help in determining the tactics of patient management.
2. Mechanism of β-Galactosidase Activation
In many studies, β-galactosidase is perceived as a tumor marker, the increased activity of which directly correlates with the presence of malignant potential in cells [16]. Oncogene activation is known to cause oncogene-induced senescence (OIS) [17]. OIS arrests the cell cycle and limits the proliferative potential of tumor cells [18]. Initially, the mechanism of senescence was studied in detail and described for somatic non-tumor cells, however, then it was later found in atypical cells. OIS results in DNA damage, which activates the p53-p21-p16 gene cascade [19]. This multi-step process overexpresses SA-β-gal [14]. As a result, this innate anticancer mechanism stops the cell cycle of atypical cells. Such cells are no longer able to enter mitosis, therefore, tumor growth stops due to the accumulation of extensive and irreversible damage to telomeric and/or non-telomeric DNA (Figure 2) [20].
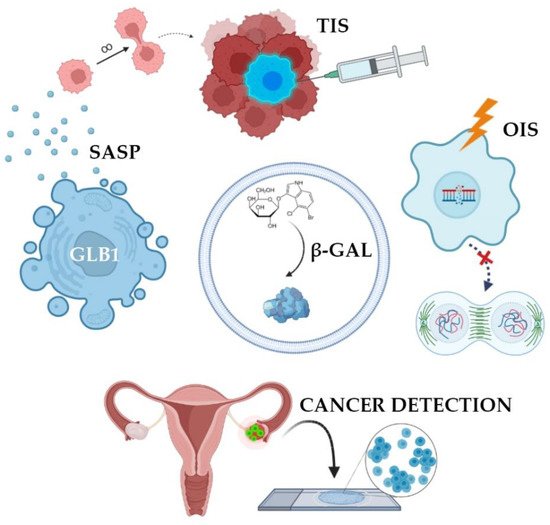
Figure 2. Clinical applications of SA-β-gal detection. SA-β-gal activity is the result of overexpression of GLB1 gene. Normally, oncogene-induced senescence (OIS) prevents cells from becoming tumors. This state can be artificially summoned in order to stop cancer growth (therapy-induced senescence, TIS). Cells accumulate SA-β-gal and acquire senescence-associated secretory phenotype. Such cells do not proliferate but produce and extract signaling molecules forcing surrounding cells into malignant transformation. Finally, some tumors and precancerous lesions have high levels of SA-β-gal, making it a potential prognostic marker and a detection probe.
3. Senescence-Associated Secretory Phenotype
Senescence may contribute to the development of cancer by altering the cellular microenvironment through the acquisition of senescence-associated secretory phenotype, SASP [18]. In this state, the cells themselves do not divide but secrete many biologically active substances, such as growth factors, chemokines, cytokines, proteases, and SA-β-gal. These substances are able to paracrinely influence neighboring cells, promoting their active proliferation [14]. SA-β-gal destroys vascular basement membranes and affects the peri-tumor environment [3], promoting tumor growth, invasion, and metastasis [21]. However, atypical cells, while expressing features of immortality, may also accumulate markers of senescence in response to treatment. SASP has been identified in tumors exposed to radiation and chemotherapeutic agents such as cisplatin, carboplatin, doxorubicin, and etoposide [22]. In theory, the negative impact of SASP components on the body can be weakened by removing senescent cells. It is worth noting that senescent cells may be subjected to mutational effects and reactivate their proliferative activity, which will lead to the emergence of various tumors. All in all, senescent mechanisms protect cells from transformation in early age, while prolonged senescence often promotes cancer [18].
4. β-Galactosidase Dependent Therapy
A major trend in oncology revolves around strategies bringing cancer cells into senescence and then triggering their selective death using senolytic therapy [13]. There are many senolytic drugs that kill senescent cells and are used as chemotherapy drugs. This therapeutic approach is called therapy-induced senescence (TIS) [22]. Senolytics selectively initiate the death of senescent cells responsible for a toxic environment around them, leading to a decrease in the number of secreted chemokines, inhibition of inflammation, and the possibility of restoring the microenvironment by neighboring cells that do not have breakdowns in the genome. To prevent further proliferation under the influence of SASP, it is necessary to eliminate these cancerous senescent cells with special senolytic agents [13]. It should be noted that senolytics are not exclusive to oncology but are also used to treat many inflammatory processes: Arthritis, dementia, heart failure. It has been proven that the activity of SA-β-gal increases in lysosomes of senescent cells, which is a target for the senolytic drug SSK1. This substance was developed on the basis of gemcitabine, a cytotoxic drug from the group of pyrimidine antagonists [23]. SSK1 is specifically activated by SA-β-gal and induces the elimination of “aged” cells. However, it has been suggested that the clinical progression of the tumor during treatment with senolytic drugs may be associated with the ability of atypical cells to avoid undergoing senescence. To solve this problem, it is proposed to combine senolytics with chemotherapy or radiation therapy.
5. SA-β-Gal Analysis in Oncopathology
The presence of senescent atypical cells has also been found in cultures of primary breast, colon, and prostate cancer cells, as well as in breast cancer, lung cancer, and melanoma cells. These findings stimulated the search for clinically valuable biomarkers of senescence in patient tissues, in particular, SA-β-gal.
As the literature data accumulated, it became obvious that the role of SA-β-gal in oncogenesis is not so simple and straightforward. Many studies indicate that it is the cells of primary tumors that contain active SA-β-gal. The activity of the studied enzyme was found in 100% of cases of primary ovarian cancer before patients underwent chemotherapy [24]. SA-β-gal levels were also measured in homogenates of ovarian tumors. In tumor tissues, the content of SA-β-gal is 50% higher compared to the content of this enzyme in healthy tissue [25]. The expression of other cellular markers of senescence was also quite significant in primary epithelial ovarian tumors, particularly phosphorylated form of histone family member X (γ-H2A.X) and tumor suppressor p53 binding protein (53BP1) [23].
In colorectal cancer cells, SA-β-gal is expressed both before and after chemotherapy. In malignant tumors of the large intestine, the level of the enzyme was two times higher than in normal tissue [26]. Colon cancer biopsies comprised “senescent sites” after treatment with 5-fluorouracil and leucvorin. The presence of such sites correlated with a higher survival rate among patients (Figure 3) [27]. In addition, serum SA-β-gal activity has been found to be elevated in invasive colon tumors [46]. SA-β-gal is also active in precancerous colon adenomas [28]. Studies in colorectal cancer have shown that TIS of tumor cells contributes to the overall outcome of chemotherapy with DNA-targeting drugs [26].
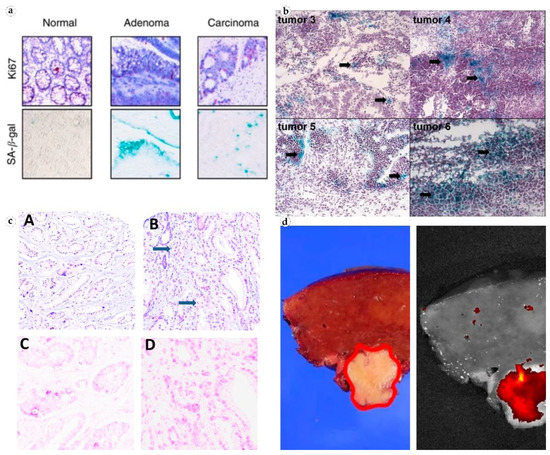
Figure 3. Visualization of SA-β-gal in patient tissues. (a) Non-senescent normal colonic crypt mucosa, senescent colon adenoma, and a formally non-senescent colon carcinoma with numerous senescent cells. Reproduced from [27] under the terms of the Creative Commons CC BY license; (b) senescent cancer cells in ovarian tumors from patients who did not receive chemotherapy. Reproduced from [24] under the terms of the Creative Commons CC BY-NC-SA license; (c) prostate cancer tissue, H&E (top), and antibodies to GLB1 (bottom). Reproduced from [33] under the terms of the Creative Commons CC BY license; (d) fluorescence imaging of β-galactosidase in freshly resected human hepatocellular carcinoma. Reproduced from [36] under the terms of the Creative Commons CC BY license.
6. SA-β-Gal Detection
New imaging techniques make it possible to detect tumor cells with high SA-β-gal content in situ. Ratiometric probes are currently being actively developed for bioluminescent imaging for cancer detecting in vivo. Activatable fluorescent probes are designed to become fluorescent only after they come in contact with the target tissue and are activated by SA-β-gal [47]. Another ratiometric probe allows visualizing SA-β-gal in colorectal cancer in real time in vivo during fluorescence endoscopy. In vivo real-time capture of β-galactose activity was performed at tumor site with a high-resolution three-dimensional view [12]. Two-photon fluorescent probe FC-β-gal was designed to visualize endogenous β-galactosidase in lysosomes in ovarian cancer cells, presenting an original approach to visualization and diagnosis of primary ovarian cancer cells [48]. Photometric imaging therapy with a macrotheranostic probe was tested to visualize cancer cells. Overexpressing SA-β-gal cells were identified through near-infrared fluorescence, photoacoustic and photothermal signals presenting new possibilities for imaging-guided cancer therapy [49]. It is also possible to detect SA-β-gal in metastatic cells by bioluminescent imaging. This approach was implemented for metastases of ovarian [50] and gastric [42] cancers.
Surprisingly, when studying SA-β-gal activity in gliomas, it was found that the activity of the enzyme was significantly higher in solid than in infiltrating tumors. The greatest increase in SA-β-gal activity was observed in oligodendroglioma, but all solid glial tumors expressed more SA-β-gal than infiltrating tumors [21]. These findings indicate that accumulation of the enzyme in tumor tissues may play a role in an innate anti-metastasis mechanism.
7. SA-β-Gal as a Prognostic Marker
SA-β-gal is active not only in tumor cells but also in cells of precancerous lesions making this enzyme a potentially valuable prognostic marker. Activity of SA-β-gal was studied in a series of breast tumors: Fibroadenoma (non-invasive benign mesenchymal tumor), proliferative fibrocystic mastopathy (which is a precancerous condition), and infiltrative breast carcinoma. The study revealed that SA-β-gal activity was normal in fibroadenoma cells, increased in cells of proliferating ducts in fibrocystic mastopathy, and at maximum level in infiltrative breast carcinoma cells [26]. These results showed a strong correlation between the levels of SA-β-gal and the malignant potential of cells.
Immunohistochemical expression of β-galactosidase in prostatic intraepithelial neoplasia of high severity (PIN III) and in glandular-stromal hyperplasia without atypia was significantly elevated. However, the level of this enzyme was lower in primary prostate cancer tissues, and minimum detection was observed in the tissue of a healthy prostate. This could be attributed to the increase in β-galactosidase activity during the early period of cell senescence which could also decrease with time. The authors also suggest that the presence of a large number of senescent cells in more highly differentiated tumors may be associated with their indolent course [31]. The activity of SA-β-gal correlated with the severity of prostatic hyperplasia in glandular-stromal hyperplasia. Prostate epithelial cells expressed SA-β-gal in patients with more pronounced prostate enlargement (mass > 55 g), while senescent cells were absent in prostates weighing <55 g [32].
8. Chemotherapy and Cellular Senescence
In breast cancer, 41% of tumors contain cells with active SA-β-gal after a course of neoadjuvant therapy. It is much higher than 2% frequency of SA-β-gal positive tumors among patients who did not undergo chemotherapy [30].
In prostate cancer tissues, β-galactosidase expression increases after neoadjuvant therapy, mainly among more clinically favorable types of moderate-severity cancers. In addition, androgen withdrawal has also been noted to induce cellular senescence in prostate tumors [33]. Given concerns about the long-term persistence of SASP cells, targeting the removal of these cells from the body may improve clinical outcomes.
The effect of chemotherapy on cellular senescence was demonstrated by studying SA-β-gal activity in lung cancer cells before and after therapy. The enzyme was not expressed in samples from patients who did not receive chemotherapy. The results were positive only in tumors excised after neoadjuvant therapy. Tumors that exhibited active SA-β-gal had reduced initial volume and retarded growth after chemotherapy [37].
9. SA-β-Gal in Non-Tumor Lesions
SA-β-gal is expressed in both malignant and benign neoplasms (i.e., in melanocytic nevi). Results of studies on SA-β-gal activity in nevi are contradicting. In the study of neonatal nevi, the enzyme was expressed in 100% of cases [38]. However, another research group proved through a rigorous methodology that adult melanocytic nevi do not contain any SA-β-gal with activity at pH 6 [39]. This difference could be attributed to differences in patient’s age or protocols which was brought up in the latter study.
There are other non-tumor pathological conditions in which active SA-β-gal is detected in tissue cells. The enzyme content in chondrocytes of articular cartilage correlates with osteoarthritis severity [40]. Moreover, SA-β-gal activity is increased in endotheliocytes of vessels affected by atherosclerosis [14].
It has been suggested that the immune response to inflammation may depend on the senescence of peripheral blood cells. It was shown that the level of SA-β-gal increased depending on the age of the donor during the cultivation of his peripheral blood mononuclear cells. The greatest age-related increase was observed in populations of CD8+ T cells, in which the proportion of cells with high SA-β-gal activity reached 64% in 60-year-old donors. CD8+ T cells with high levels of SA-β-gal had shortened telomeres and upregulated p16 pathway. This led to a disruption of proliferation and differentiation in T cells [53].
2.9. SA-β-Gal in Fibrosis
A number of studies report correlations between SA-β-gal expression in fibroblasts and the severity of fibrotic processes in connective tissue. Similar to tumors, senescent fibroblasts gradually increase p53, p21WAF1, and p16INK4a levels, produce high levels of reactive oxygen species, and accumulate oxidative DNA damage [54,55]. Gradually this leads to overproduction of collagen and fibrotic transformation of the tissue. It is also possible that the ability of serum growth factors to modulate senescent cells can be connected with the upregulation of collagenase synthesis. After such stimulation, the culture of “young” fibroblasts responded with an increase in the levels of procollagenase and tissue inhibitor of metalloproteinases. However, senescent fibroblasts produced relatively high levels of procollagenase in low serum concentrations, did not increase procollagenase synthesis, and expressed TIMP at low levels [56]. Other authors showed that β-galactosidase was present in senescent fibroblasts and keratinocytes but was absent in resting fibroblasts and terminally differentiated keratinocytes. An increase in this marker in fibroblasts and epidermal keratinocytes depended on the age of the donor [5]. When studied in vitro, fibroblasts excised from elderly people had higher SA-β-gal activity compared to fibroblasts from younger people. Similar results were obtained when assessing age dependence of cell apoptosis [57].
Researchers from China investigated the effect of cigarette smoke extract on the growth, proliferation, and aging of skin fibroblasts and the possible mechanism underlying these effects. Fibroblasts exposed to even low concentrations of cigarette smoke for a long period of time (5 passages) showed significantly increased SA-β-gal activity and typical signs of “aging” cells [58].
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12102309
This entry is offline, you can click here to edit this entry!
