Immunological checkpoint inhibitors (ICPI) are monoclonal antibodies that target the signaling pathways of the immune system that involve the programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) molecules, contributing to restore the immune responses against neoplastic cells.
- atezolizumab
- avelumab
- cemiplimab
- durvalumab
- ipilimumab
- nivolumab
- pembrolizumab
1. Introduction
The drugs called immunological checkpoint inhibitors (ICPI) have revolutionized the treatment of several oncological pathologies. These monoclonal antibodies target the signaling pathways of the immune system that involve the programmed cell death protein 1 (PD-1), programmed cell death ligand 1 (PD-L1), and cytotoxic T-lymphocyte antigen 4 (CTLA-4) molecules, contributing to restore the immune responses against neoplastic cells [2].
Despite the important clinical benefits of immunotherapy with the use of ICPI, including increased overall survival, these drugs are associated with significant adverse events related to the immune system, which can affect any organ or system of the body, the most frequently being gastrointestinal tract, endocrine glands, skin, and liver [1,2,3].
2. Immunological Checkpoint Inhibitors Available in the Pharmaceutical Market and Main Associated Adverse Effects
ICPI lead to an increase in the anti-tumor activity of the immune system by blocking the intrinsic negative regulators of immunity, such as the CTLA-4 receptor (cytotoxic T-lymphocyte antigen 4) of cytotoxic T lymphocytes, the PD-1 receptor (programmed cell death protein 1) of cytotoxic T lymphocytes, or their PD-L1 ligand (programmed cell death ligand 1) in tumor cells [1,2]. Thus, ICPI inhibit the connection between the aforementioned cytotoxic T lymphocyte receptors and the corresponding cell ligands neoplastic cells, preventing the development of tolerance by cytotoxic T lymphocytes and, consequently, preventing neoplastic cells from escaping cell death mediated by those lymphocytes (Figure 1) [1,2]. It should be noted that under normal conditions (i.e., in the absence of oncological pathology or in tissues without oncological pathology), the aforementioned ligands are presented to cytotoxic T lymphocytes by antigen-presenting cells (APC), which allows the development of tolerance for the body’s own cells.
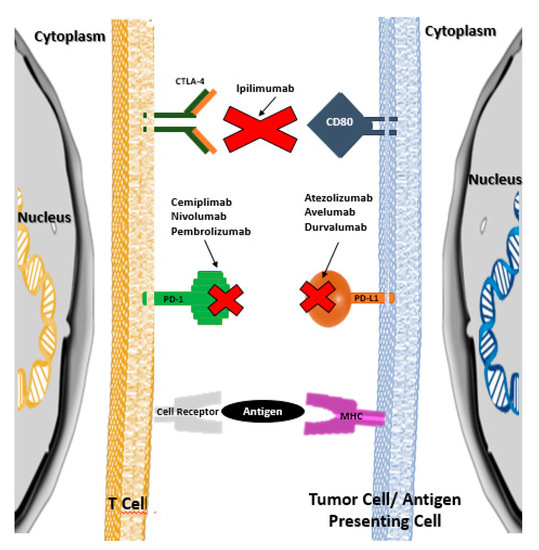
Figure 1. Therapeutic targets of immunological checkpoint inhibitors (adapted from references [1] and [2]).
In several neoplastic pathologies, ICPI have led to an increase in the overall survival of cancer patients. Table 1 shows the various ICPI approved in July 2020 by the U.S. Food and Drug Administration (U.S. FDA) and the European Medicines Agency (EMA), as well as their mechanism of action.
Table 1. Checkpoints inhibitors approved by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA) presented according to the chronological order of introduction into the pharmaceutical market and their mechanism of action.
| ICPI Monoclonal Antibody | Trade Name | Mechanism of Action |
|---|---|---|
| Ipilimumab | Yervoy | CTLA-4 inhibitor |
| Pembrolizumab | Keytruda | PD-1 inhibitor |
| Nivolumab | Opdivo | PD-1 inhibitor |
| Atezolizumab | Tecentriq | PD-L1 inhibitor |
| Avelumab | Bavencio | PD-L1 inhibitor |
| Durvalumab | Imfinzi | PD-L1 inhibitor |
| Cemiplimab | Libtayo | PD-1 inhibitor |
CTLA-4: Cytotoxic T-lymphocyte antigen 4; ICPI: Immune checkpoint inhibitors; PD-1: Programmed cell death 1; PD-L1: Programmed cell death ligand 1.
Table 2 shows the authorized therapeutic indications of the ICPI available in the pharmaceutical market.
Table 2. The authorized therapeutic indications of the ICPI available in the pharmaceutical market.
| Ipilimu. | Pembrolizu. | Nivolu. | Atezolizu. | Avelu. | Durvalu. | Cemipli. | |
|---|---|---|---|---|---|---|---|
| Breast cancer | ✓ | ||||||
| Cervical cancer | ✓ | ||||||
| Colorectal cancer | ✓ | ✓ | ✓ | ||||
| Cutaneous squamous cell carcinoma | ✓ | ✓ | |||||
| Endometrial carcinoma | ✓ | ||||||
| Esophageal cancer | ✓ | ✓ | |||||
| Gastric cancer | ✓ | ||||||
| Head and neck cancer | ✓ | ✓ | |||||
| Hepatocellular carcinoma | ✓ | ✓ | ✓ | ✓ | |||
| Hodgkin lymphoma | ✓ | ✓ | |||||
| Large B-cell lymphoma | ✓ | ||||||
| Melanoma | ✓ | ✓ | ✓ | ✓ | |||
| Merkel cell carcinoma | ✓ | ✓ | |||||
| Microsatellite instability-high tumors | ✓ | ||||||
| Non-small cell lung cancer | ✓ | ✓ | ✓ | ✓ | ✓ | ||
| Renal cell carcinoma | ✓ | ✓ | ✓ | ✓ | |||
| Small cell lung cancer | ✓ | ✓ | ✓ | ✓ | |||
| Tumor mutation burden-high tumors | ✓ | ||||||
| Urothelial carcinoma | ✓ | ✓ | ✓ | ✓ | ✓ |
Cancer patients who receive ICPI may experience a specific set of adverse drug reactions, which are often referred to as immune-related adverse events (irAEs) [4,5]. These adverse reactions may involve several organs from different systems of the organism (Figure 2), and although they are generally mild in intensity, serious, irreversible, or even potentially fatal, adverse reactions can sometimes occur [1,2,6]. Since ICPI therapies are relatively recent, few doctors have clinical experience in recognizing and treating the adverse effects associated with these drugs.
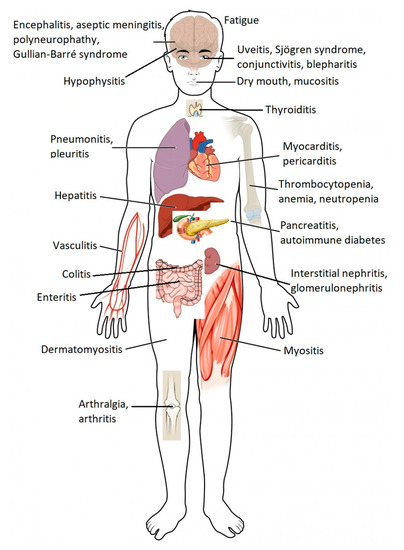
Figure 2. Common immune-related adverse events associated with immune checkpoint blockade by organs of the human body. Immune checkpoint blockade can result in the inflammation of any organ (adapted from ref. [1,6]).
The main adverse effects associated with ICPI are shown in Table 3, and more detailed information is presented in Table S1. Since there are no prospective clinical studies that have defined strategies for the proper monitoring of adverse reactions related to the immune system, clinical practice in this area has been revealed as quite variable.
Table 3. Most common adverse effects associated with ICPI (adapted from ref. [4]).
| Affected Organ or System of the Human Body | Immune Related Adverse Effects |
|---|---|
| Skin | Bullous dermatosis Skin rash/Inflammatory dermatitis Severe skin reactions |
| Gastrointestinal | Colitis Hepatitis |
| Lung | Pneumonitis |
| Endocrine | Diabetes Hyperthyroidism (primary) Hypophysitis Primary adrenal insufficiency |
| Musculoskeletal | Inflammatory arthritis Myositis Polymyalgia rheumatica |
| Renal | Nephritis |
| Nervous System | Myasthenia gravis Guillain–Barré syndrome Peripheral neuropathy Autonomic neuropathy Aseptic meningitis Encephalitis Transverse myelitis |
| Hematological | Autoimmune hemolytic anemia Acquired thrombotic thrombocytopenic purpura Uremic hemolytic syndrome Aplastic anemia Lymphopenia Immune thrombocytopenia Acquired hemophilia |
| Cardiovascular | Myocarditis Pericarditis ArrhythmiasHeart failure associated with ventricular failure Vasculitis Venous thromboembolism |
| Ocular | Uveitis/Iritis Episcleritis Blepharitis |
Several recommendations have been published to assist doctors and other health professionals to diagnose and identify adverse reactions related to the immune system associated with ICPI. These recommendations have also been aimed at harmonizing the monitoring of adverse reactions associated with ICPI. Examples of these recommendations are the guidelines of the European Society for Medical Oncology (ESMO) [3], the guidelines of the Society for Immunotherapy of Cancer (SITC) [7] and, more recently, the guidelines of the National Cancer Control Network (NCCN) [5], which were developed in collaboration with the American Society of Clinical Oncology (ASCO). More recently, in June 2018, ASCO published guidelines for clinical practice that address the adverse effects associated with ICPI—which are related to the immune system, based on the various systems or organs of the human body—together with recommendations for the diagnostic investigation and respective monitoring/treatment [4]. The development of these latest guidelines involved a systematic review of the literature and a consensus process, which was obtained from a multidisciplinary and multi-organizational panel of experts, consisting of doctors of various specialties, nurses, and specialists in clinical trials and advocacy [4].
The wide range of adverse drug reactions, involving the immune system, associated with ICPI, requires a synergistic multidisciplinary approach, involving several health professionals, for their monitoring, with the hospital pharmacist playing an important role in this multidisciplinary team, with a view to preventing and minimizing the adverse drug reactions mentioned.
It is also important to highlight that the complexity and multifactorial characteristics of neoplastic diseases require a molecular pathological epidemiology (MPE) approach [8]. The exposure to different risk factors influences specific carcinogenic mechanisms and different degrees of carcinogenicity. The integration of microbiology in the MPE model helps healthcare professionals and researchers understand the complex interactions existing between tumor cells, the immune system, and microorganisms in the tumor microenvironment during the carcinogenic process [9]. In order to optimize the effectiveness and safety of immunotherapies directed to immunological checkpoints, it is also essential to investigate and evaluate the influence of several immunomodulatory factors (such as environmental, dietary, lifestyle, microbial and pharmacological factors) in the response to immunotherapy.
This entry is adapted from the peer-reviewed paper 10.3390/vaccines8040575
