Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The diagnosis of SARS-CoV-2 pneumonia is expected to worsen, and mortality will be higher when combined with myocardial injury (MI). The combination of novel coronavirus infections in patients with MI can cause confusion in diagnosis and assessment, with each condition exacerbating the other, and increasing the complexity and difficulty of treatment.
- myocardial injury
- SARS-CoV-2
- management
- pathogenesis
1. Mechanism of MI Related to SARS-CoV-2 Pneumonia
In patients with COVID-19 infection, the heart requires special attention. The results of histopathology in COVID-19 patients showed that the infiltrating virus particles were visible in the myocardial tissue. Still, there was no substantial myocardial damage, suggesting that the virus may not directly injure the myocardium [1]. The pathophysiological mechanism of myocardial injury (MI) associated with SARS-CoV-2 pneumonia is still controversial (Figure 1). The pathogenesis of MI may include damage to the heart muscle through direct and/or indirect action. The direct damage occurs when the virus infects the heart muscle cells by recognizing the ACE2 receptor, while the immune response may cause indirect damage. The critical component of the immune response to SARS-CoV-2 infection in macrophages is the overproduction of inflammatory cytokines such as tumor necrosis factor alpha (TNF-α) [2]. When COVID-19 infects a cell, ACE2 on the cell surface is internalized, reducing the receptor density. [3]. The reduction in surface ACE2 causes an accumulation of Ang II [4]. Furthermore, the overactivation of ADAM-17 due to the increase in Ang II binding to the Ang II type I receptors reduces the Ang II clearance, increasing the Ang II-mediated inflammatory response [3][4]. Since ACE2 receptors are involved in the pathophysiology of COVID-19, the use of renin–angiotensin–aldosterone system inhibitors for protection against COVID-19-related cardiovascular symptoms remains controversial and debated.
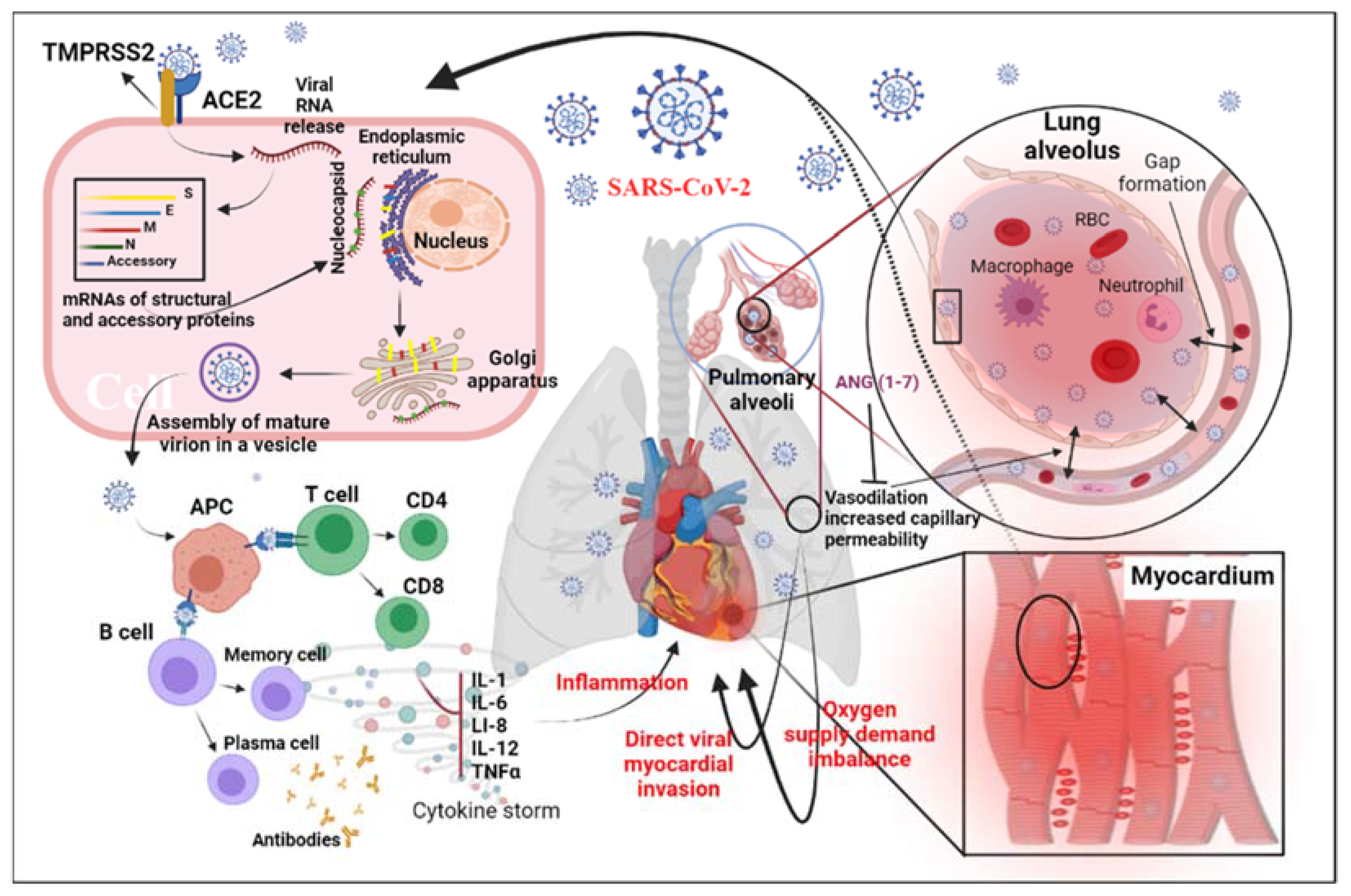
Figure 1. Diagram of potential physiological mechanisms of MI related to SARS-CoV-2: This figure shows the proposed mechanisms of MI related to SARS-CoV-2 infection through direct viral infection, inflammatory factors, and/or imbalance of the oxygen supply caused by acute respiratory distress syndrome. Abbreviations: ACE2, angiotensin-converting enzyme 2; S, spike; E, envelope; M, matrix/membrane, N, nucleocapsid; ANG, angiotensin; APC, antigen-presenting cell; IL-1, interleukin 1; IL-6, interleukin 6; IL-12, interleukin 12; TNF-α, tumor necrosis factor alpha; TMPRSS2, transmembrane protease, serine 2.
Whole-genome sequence analysis results show that the spike (S) protein encoded by SARS-CoV-2 contains similar ACE2 receptor-binding domains (S1) [5][6]. Audit et al., studying heart samples from coronavirus-infected mice and SARS-CoV-2 patients who died, found that SARS-CoV-2 infection in the lungs of mice could lead to ACE2-dependent MI [7]. At the same time, they found that SARS-CoV-2 RNA could be detected in 35% of heart samples of patients who died of SARS [7]. The research results from several teams also showed that SARS-CoV-2 infection, expression, and transcription are associated with ACE2 [8][9]. The aforementioned studies indicate that SARS-CoV-2 can damage myocardial cells by infecting the ACE2 receptors.
Studies have shown that cellular inflammatory factors result from an imbalance of TH1 and TH2 cytokine interactions in SARS-CoV-2 patients, and that levels of the inflammatory factors IL-4, IL-10, and IL-6 in tissue samples are elevated [10][11]. The high levels of serum cytokines—including IL-6, IL-1, IL-8, IL-12, and TNF-α—are reported to be associated with severe acute respiratory syndrome (SARS) in coronavirus infection [12][13][14][15]. Recently, research has shown that excessive T-cell activation in the peripheral blood of SARS-CoV-2 patients causes increased TH17 and high toxicity of CD8 T cells [1]. The aforementioned studies indicate that SARS-CoV-2 patients suffer from severely exaggerated immune responses.
Inflammatory factors may be involved in the process of heart failure [16]. It has been suggested that other patterns of heart muscle damage differ from those caused by direct infection with the virus. Similar reports on patients infected with the SARS-CoV-2 virus show that SARS-CoV-2 patients suffer from reversible diastolic damage related to inflammatory factors [17]. Therefore, more research on anti-inflammatory cytokines may reveal the pathogenesis of SARS-CoV-2 and inhibit inflammatory factors that may reverse heart muscle damage.
The severe symptoms that can be associated with COVID-19 infection have a significant impact on the cardiac muscle. For example, hypoxemia, respiratory distress syndrome, shock, or hypotension induced by lung infection could lead to insufficient oxygen supply to the myocardium. Furthermore, among patients with cardiac impairment or with chronic cardiovascular diseases such as coronary heart disease, the impact of these symptoms is more severe due to the increased stress on the heart and the lack of capacity to meet the required demand [18]. In addition, previous studies show that up to 20% of SARS-CoV-2 patients have an abnormal clotting function caused by MI [10][11][19]. However, the causal relationship between thrombosis and MI needs further clinical observation and clarification of pathological findings.
Elevated troponin is one of the clinical manifestations of acute MI. However, it is necessary to pay attention to its compatibility with the clinical phenotype, since troponin elevation is affected by various disease conditions [20]. Previous reports have shown that increased troponin levels may also occur after mechanical stretching induced by preload or normal cardiac physiological stress [20]. The autopsy reports of COVID-19 patients also show a small amount of infiltration of inflammatory cells in the patients’ heart tissue, with no other significant damage [1]. Therefore, further evidence is needed to support the use of troponin as a marker of direct heart injury in patients with SARS-CoV-2.
2. Management and Treatment of MI Related to SARS-CoV-2 Pneumonia
Generally, SARS-CoV-2 infection leads to pneumonia, associated with muscle fatigue, fever, and dry cough. Although these symptoms present as the first clinical manifestations of the infection in most patients, cardiac symptoms such as palpitations, chest tightness, and chest pain have been reported among SARS-CoV-2 pneumonia patients [2].
A significant decline has been reported in myocardial injuries, attributed to patients’ fear of visiting hospitals during the pandemic [21]. Huet et al. noted a significant decline in cases of myocardial infarction and heart failure during the SARS-CoV-2 lockdown compared to the number of cases reported before the lockdown (4.8 ± 1.6 vs. 2.6 ± 1.5 patients per day, p = 0.0006) [22].
A growing body of evidence has indicated that cardiac injury is common among COVID-19 patients and is associated with the severity of the disease. A meta-analysis has shown that out of 1527 SARS-CoV-2 pneumonia patients, at least 8% suffered from acute cardiac injury; furthermore, they found that SARS-CoV-2 pneumonia patients with more severe symptoms have 13 times the risk of cardiac injuries as compared to asymptomatic patients [23][24][25]. Acute myocardial infarction is a life-threatening condition that is characterized by increased high-sensitivity cardiac troponin (hs-cTn) or abnormalities in the patient’s ECG (e.g., ST elevation) [26]. According to Zeng et al. and Weltz et al., confirmed coronary revascularization for a SARS-CoV-2 pneumonia patient with ST-elevation myocardial infarction (STEMI) should be considered after evaluating the risks and benefits based on the patient’s state, with the potential to explore fibrinolytic therapy rather than percutaneous coronary intervention (PCI) [27][28][29]. However, Cameli et al. reported an increased risk of disseminating intravascular coagulation (DIC) and hemorrhagic complications associated with fibrinolytic treatment [26].
The management of cardiac patients with SARS-CoV-2 pneumonia can be challenging, and such cases may need additional care because of the higher thrombus burden. In a case series from Italy, even though the authors tried to limit PCI treatment to the severe culprit lesions and delay the non-culprit lesions until the patients’ recovery from SAR-CoV-2 pneumonia, all SARS-CoV-2 pneumonia patients with acute coronary syndromes (ST-elevation myocardial infarction (STEMI), non-ST elevation myocardial infarction (NSTEMI), and Takotsubo syndrome (TTS)) underwent angiography and were eventually treated invasively [30]. Furthermore, all subjects received dual antiplatelet therapy with ticagrelor–aspirin, except for four subjects who received clopidogrel–aspirin. cPAP ventilation was required as respiratory support for all patients except for six, who needed endotracheal intubation (ETI), following the PCI. Herein lies the importance of using ticagrelor and clopidogrel [31][32]. In a prospective study, Choudry et al. noted that SARS-CoV-2 patients had higher levels of troponin, D-dimer protein, and C-reactive protein (1221 ng/L vs. 369 ng/L, p = 0.0028; 1.86 mg/L vs. 0.52 mg/L, p = 0.0012; and 12 mg/L vs. 50 mg/L, p = 0.01, respectively), while their lymphocyte counts were lower (1.3 109/L vs. 1.7 109/L, p = 0.0002), compared to non-SARS-CoV-2 patients [33]. Moreover, significantly higher multivessel thrombogenicity and incidence of stent thrombosis were observed, and a significantly lower left ventricular ejection fraction was detected, among SARS-CoV-2 pneumonia patients. Furthermore, a higher rate of comorbidities was detected among SARS-CoV-2 pneumonia patients, including diabetes mellitus, arterial hypertension, and hyperlipidemia.
Additionally, no significant difference was observed between groups in the total dose of heparin (SARS-CoV-2 11125 U vs. non-SARS-CoV-2 10066 U, p = 0.15), and similar average activated clotting time (ACT) was achieved during the procedures (p = 0.261); however, SARS-CoV-2 pneumonia patients needed a longer hospital stay, and were more likely to be admitted to the intensive care unit (p = 0.004) [33]. In a study by Bangalore et al., all 18 SARS-CoV-2 patients were reported to have elevated D-dimer and ST-segment elevation. In addition, a high prevalence of non-obstructive injuries and a poor prognosis were observed [34].
Fibrinolytic agents such as alteplase and tenecteplase were used in an international study; the authors reported successful fibrinolysis in 50 (85%) patients out of 59, with a median reperfusion time of 27 minutes [35]. Of the nine failed cases, six recovered after PCI; one died before the procedure, and two after it. Nineteen procedures were performed without using the fibrinolytic agents, and the total number of performed PCI/coronary artery bypass graft (CABG)/drug-eluting stent (DES) procedures was 28, representing 36% of COVID-19 cases, which is less than in other studies. Chinese experts recommend using fibrinolytic agents in stable patients who present to the ER within less than 12 h of the onset of symptoms and do not have any contraindications for this class of medicines [27][29]. However, many questions remain unanswered about the characteristics and indicators of the suitable populations for this approach, along with the strategies adopted and the long-term impacts or results.
Finally, Akşit E. suggested the use of ticagrelor for a patient with myocardial infarction during the pandemic for three reasons: (1) because of its pleiotropic effects, there is a lower risk due to the decreased levels of pro-inflammatory markers and the suppressed activation of platelets via the A2A and A2B adenosine receptors, reducing the chance of DIC; (2) ticagrelor showed a potential to reduce thromboinflammatory biomarkers; and (3) recent research shows that it has antibiotic potential against Gram-positive bacteria, which might increase the chances of survival in patients with coexisting diseases [36][37][38].
The histological and pathophysiological effects of COVID-19 on the heart muscle remain unclear and controversial. A histopathological analysis showed that the virus could infiltrate the cardiac muscle by utilizing the ACE2 receptor. However, the gross examination of the hearts of 51 patients showed that aside from the expected findings (i.e., mild pericardial edema and some serosanguinous pericardial effusion) from pre-existing conditions such as coronary heart disease in 29 cases, no notable abnormalities were found [39]. Furthermore, although a small number of case reports have shown that SARS-CoV-2 pneumonia can infect the myocardium, leading to viral myocarditis, the damage in the vast majority of cases was caused by increased cardiometabolic demand because of the systemic infection and ongoing hypoxia caused by severe pneumonia or ARDS [40]. Kawakami et al. concluded that the mechanism by which the virus causes cardiac damage remains uncertain, and that the infiltration by macrophages and T cells can be seen in noninfectious deaths [41].
This entry is adapted from the peer-reviewed paper 10.3390/jcdd9090307
References
- Xu, Z.; Shi, L.; Wang, Y.; Zhang, J.; Huang, L.; Zhang, C.; Liu, S.; Zhao, P.; Liu, H.; Zhu, L. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir. Med. 2020, 8, 420–422.
- Peng, W.; Wu, H.; Tan, Y.; Li, M.; Yang, D.; Li, S. Mechanisms and treatments of myocardial injury in patients with corona virus disease 2019. Life Sci. 2020, 262, 118496.
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S. Renin–angiotensin–aldosterone system inhibitors and risk of Covid-19. N. Engl. J. Med. 2020, 382, 2441–2448.
- Gheblawi, M.; Wang, K.; Viveiros, A.; Nguyen, Q.; Zhong, J.-C.; Turner, A.J.; Raizada, M.K.; Grant, M.B.; Oudit, G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: Celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020, 126, 1456–1474.
- Li, W.; Moore, M.J.; Vasilieva, N.; Sui, J.; Wong, S.K.; Berne, M.A.; Somasundaran, M.; Sullivan, J.L.; Luzuriaga, K.; Greenough, T.C. Angiotensin-converting enzyme 2 is a functional receptor for the SARS coronavirus. Nature 2003, 426, 450–454.
- Chaudhary, M. COVID-19 susceptibility: Potential of ACE2 polymorphisms. Egypt. J. Med. Hum. Genet. 2020, 21, 54.
- Oudit, G.; Kassiri, Z.; Jiang, C.; Liu, P.; Poutanen, S.; Penninger, J.; Butany, J. SARS-coronavirus modulation of myocardial ACE2 expression and inflammation in patients with SARS. Eur. J. Clin. Investig. 2009, 39, 618–625.
- Wan, Y.; Shang, J.; Graham, R.; Baric, R.S.; Li, F. Receptor recognition by the novel coronavirus from Wuhan: An analysis based on decade-long structural studies of SARS coronavirus. J. Virol. 2020, 94, e00127-20.
- Zhou, P.; Yang, X.-L.; Wang, X.-G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.-R.; Zhu, Y.; Li, B.; Huang, C.-L. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273.
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506.
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513.
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Liu, H.; Wu, Y.; Zhang, L.; Yu, Z.; Fang, M.; Yu, T. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481.
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.; Ross, R.; Frydas, I.; Kritas, S. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost. Agents 2020, 34, 327–331.
- Channappanavar, R.; Perlman, S. Pathogenic human coronavirus infections: Causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017, 39, 529–539.
- Wong, C.; Lam, C.; Wu, A.; Ip, W.; Lee, N.; Chan, I.; Lit, L.; Hui, D.; Chan, M.; Chung, S. Plasma inflammatory cytokines and chemokines in severe acute respiratory syndrome. Clin. Exp. Immunol. 2004, 136, 95–103.
- Mann, D.L. Inflammatory mediators and the failing heart: Past, present, and the foreseeable future. Circ. Res. 2002, 91, 988–998.
- Li, S.S.; Cheng, C.; Fu, C.; Chan, Y.; Lee, M.; Chan, J.W.; Yiu, S. Left ventricular performance in patients with severe acute respiratory syndrome: A 30-day echocardiographic follow-up study. Circulation 2003, 108, 1798–1803.
- Musher, D.M.; Abers, M.S.; Corrales-Medina, V.F. Acute infection and myocardial infarction. New Engl. J. Med. 2019, 380, 171–176.
- Marfella, R.; Paolisso, P.; Sardu, C.; Palomba, L.; D’Onofrio, N.; Cesaro, A.; Barbieri, M.; Rizzo, M.R.; Sasso, F.C.; Scisciola, L. SARS-COV-2 colonizes coronary thrombus and impairs heart microcirculation bed in asymptomatic SARS-CoV-2 positive subjects with acute myocardial infarction. Crit. Care 2021, 25, 217.
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Chaitman, B.R.; Bax, J.J.; Morrow, D.A.; White, H.D.; Mickley, H.; Crea, F.; Van de Werf, F. Fourth universal definition of myocardial infarction (2018). Eur. Heart J. 2019, 40, 237–269.
- Metzler, B.; Siostrzonek, P.; Binder, R.K.; Bauer, A.; Reinstadler, S.J. Decline of acute coronary syndrome admissions in Austria since the outbreak of COVID-19: The pandemic response causes cardiac collateral damage. Eur. Heart J. 2020, 41, 1852–1853.
- Huet, F.; Prieur, C.; Schurtz, G.; Gerbaud, É.; Manzo-Silberman, S.; Vanzetto, G.; Elbaz, M.; Tea, V.; Mercier, G.; Lattuca, B. One train may hide another: Acute cardiovascular diseases could be neglected because of the COVID-19 pandemic. Arch. Cardiovasc. Dis. 2020, 113, 303–307.
- Li, B.; Yang, J.; Zhao, F.; Zhi, L.; Wang, X.; Liu, L.; Bi, Z.; Zhao, Y. Prevalence and impact of cardiovascular metabolic diseases on COVID-19 in China. Clin. Res. Cardiol. 2020, 109, 531–538.
- Maestrini, V.; Birtolo, L.I.; Francone, M.; Galardo, G.; Galea, N.; Severino, P.; Alessandri, F.; Colaiacomo, M.C.; Cundari, G.; Chimenti, C. Cardiac involvement in consecutive unselected hospitalized COVID-19 population: In-hospital evaluation and one-year follow-up. Int. J. Cardiol. 2021, 339, 235–242.
- Gragnano, F.; Cesaro, A.; Pelliccia, F.; Calabrò, P. Multimodality evaluation of cardiac injury in COVID-19: Getting to the heart of the matter. Int. J. Cardiol. 2021, 339, 243–245.
- Cameli, M.; Pastore, M.C.; Mandoli, G.E.; D’ascenzi, F.; Focardi, M.; Biagioni, G.; Cameli, P.; Patti, G.; Franchi, F.; Mondillo, S. COVID-19 and Acute Coronary Syndromes: Current Data and Future Implications. Front. Cardiovasc. Med. 2020, 7, 593496.
- Zeng, J.; Huang, J.; Pan, L. How to balance acute myocardial infarction and COVID-19: The protocols from Sichuan Provincial People’s Hospital. Intensive Care Med. 2020, 46, 1111–1113.
- Welt, F.G.; Shah, P.B.; Aronow, H.D.; Bortnick, A.E.; Henry, T.D.; Sherwood, M.W.; Young, M.N.; Davidson, L.J.; Kadavath, S.; Mahmud, E. Catheterization laboratory considerations during the coronavirus (COVID-19) pandemic: From the ACC’s Interventional Council and SCAI. J. Am. Coll. Cardiol. 2020, 75, 2372–2375.
- Akkaif, M.A.; Sha’aban, A.; Cesaro, A.; Jaber, A.A.S.; Vergara, A.; Yunusa, I.; Jatau, A.I.; Mohammed, M.; Govindasamy, G.S.; Al-Mansoub, M.A. The impact of SARS-CoV-2 treatment on the cardiovascular system: An updated review. Inflammopharmacology 2022, 30, 1143–1151.
- Secco, G.G.; Tarantini, G.; Mazzarotto, P.; Garbo, R.; Parisi, R.; Maggio, S.; Vercellino, M.; Pistis, G.; Audo, A.; Kozel, D. Invasive strategy for COVID patients presenting with acute coronary syndrome: The first multicenter Italian experience. Catheter. Cardiovasc. Interv. 2021, 97, 195–198.
- Akkaif, M.A.; Daud, N.A.A.; Sha’aban, A.; Ng, M.L.; Abdul Kader, M.A.S.; Noor, D.A.M.; Ibrahim, B. The Role of Genetic Polymorphism and Other Factors on Clopidogrel Resistance (CR) in an Asian Population with Coronary Heart Disease (CHD). Molecules 2021, 26, 1987.
- Akkaif, M.A.; Sha’aban, A.; Daud, N.A.A.; Yunusa, I.; Ng, M.L.; Kader, M.A.S.A.; Noor, D.A.M.; Ibrahim, B. Coronary Heart Disease (CHD) in Elderly Patients: Which Drug to Choose, Ticagrelor and Clopidogrel? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Cardiovasc. Dev. Dis. 2021, 8, 123.
- Choudry, F.A.; Hamshere, S.M.; Rathod, K.S.; Akhtar, M.M.; Archbold, R.A.; Guttmann, O.P.; Woldman, S.; Jain, A.K.; Knight, C.J.; Baumbach, A. High thrombus burden in patients with COVID-19 presenting with ST-segment elevation myocardial infarction. J. Am. Coll. Cardiol. 2020, 76, 1168–1176.
- Bangalore, S.; Sharma, A.; Slotwiner, A.; Yatskar, L.; Harari, R.; Shah, B.; Ibrahim, H.; Friedman, G.H.; Thompson, C.; Alviar, C.L. ST-segment elevation in patients with Covid-19—a case series. New Engl. J. Med. 2020, 382, 2478–2480.
- Hamadeh, A.; Aldujeli, A.; Briedis, K.; Tecson, K.M.; Sanz-Sánchez, J.; Al-Obeidi, A.; Diez, J.L.; Žaliūnas, R.; Stoler, R.C.; McCullough, P.A. Characteristics and outcomes in patients presenting with COVID-19 and ST-segment elevation myocardial infarction. Am. J. Cardiol. 2020, 131, 1–6.
- Akşit, E.; Kırılmaz, B.; Gazi, E.; Aydın, F. Ticagrelor can be an important agent in the treatment of severe COVID-19 patients with myocardial infarction. Balk. Med. J. 2020, 37, 233.
- Akkaif, M.A.; Ng, M.L.; Kader, M.A.S.A.; Daud, N.A.A.; Sha’aban, A.; Ibrahim, B. A review of the effects of ticagrelor on adenosine concentration and its clinical significance. Pharmacol. Rep. 2021, 73, 1551–1564.
- Akkaif, M.A.; Sha’aban, A.; Daud, N.A.A.; Ng, M.L.; Ibrahim, B. Investigate the Strategy of Using Pharmacogenetics and Pharmacometabonomics to the Personalization of Ticagrelor Antiplatelet Therapy. Syst. Rev. Pharm. 2020, 11, 1100–1107.
- Schaefer, I.-M.; Padera, R.F.; Solomon, I.H.; Kanjilal, S.; Hammer, M.M.; Hornick, J.L.; Sholl, L.M. In situ detection of SARS-CoV-2 in lungs and airways of patients with COVID-19. Mod. Pathol. 2020, 33, 2104–2114.
- Thallapureddy, K.; Thallapureddy, K.; Zerda, E.; Suresh, N.; Kamat, D.; Rajasekaran, K.; Moreira, A. Long-Term Complications of COVID-19 Infection in Adolescents and Children. Curr. Pediatr. Rep. 2022, 10, 11–17.
- Kawakami, R.; Sakamoto, A.; Kawai, K.; Gianatti, A.; Pellegrini, D.; Nasr, A.; Kutys, B.; Guo, L.; Cornelissen, A.; Mori, M. Pathological evidence for SARS-CoV-2 as a cause of myocarditis: JACC review topic of the week. J. Am. Coll. Cardiol. 2021, 77, 314–325.
This entry is offline, you can click here to edit this entry!
