The anatomical elements that in humans guard the unidirectional blood flow from the cardiac ventricles to the aortic and pulmonary arteries are the arterial (semilunar) valves. The valve that prevents blood backflow from the aorta to the left ventricle is the aortic valve, while that which performs this function between the pulmonary artery and the right ventricle is the pulmonary or pulmonic valve. The main medical interest in arterial valves is that their congenital malformations and diseases over a lifetime are clinically relevant. Although both valves are subject to similar complications, those affecting the aortic valve cause the most severe effects.
- heart
- outflow tract valves
- vertebrates
- medical concept
1. The Cardiac Outflow Tract Valves of Chondrichthyans and Actinopterygians
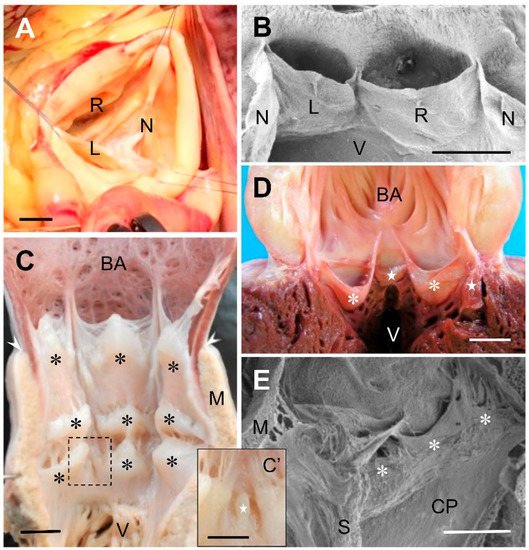
2. The Cardiac Outflow Tract Valves of Early Sarcopterygians
3. The Cardiac Outflow Tract Valves of Reptiles
4. The Cardiac Outflow Tract Valves of Birds and Mammals
This entry is adapted from the peer-reviewed paper 10.3390/jcdd9100318
References
- Sans-Coma, V.; Gallego, A.; Muñoz-Chápuli, R.; De Andrés, A.V.; Durán, A.C.; Fernández, B. Anatomy and histology of the cardiac conal valves of the dogfish (Scyliorhinus canicula). Anat. Rec. 1995, 241, 496–504.
- Grimes, A.C. The cardiac outflow tract of primitive fishes. In Phylogeny, Anatomy and Physiology of Ancient Fishes; Zaccone, G., Dabrowski, K., Hedrick, M.S., Fernandes, J.M.O., Icardo, J.M., Eds.; CRC Press: Boca Raton, FL, USA, 2016; pp. 151–178.
- White, E. The heart valves of the Elasmobranch fishes. Am. Mus. Novitatis 1936, 838, 1–21.
- Satchell, G.H.; Jones, M.P. The function of the conus arteriosus in the Port Jackson shark, Heterodontus portusjacksoni. J. Exp. Biol. 1967, 46, 373–382.
- Anthony, J.; Millot, J.; Robineau, D. Le coeur et l’aorte ventrale de Latimeria chalumnae (Poisson coelacanthidé). Comptes Rendus De L’academie Des Sci., Paris 1965, 261, 223–226.
- Robertson, J.I. The development of the heart and vascular system of Lepidosiren paradoxa. Q. J. Microsc. Sci. 1913, 59, 53–132.
- Burggren, W.W.; Johansen, K. Circulation and respiration in lungfishes (Dipnoi). J. Morphol. 1986, 190, 217–236.
- Szidon, J.P.; Lahiri, S.; Lev, M.; Fishman, A.P. Heart and circulation of the African lungfish. Circ. Res. 1969, 25, 23–38.
- Icardo, J.M.; Brunelli, E.; Perrotta, I.; Colvee, E.; Wong, W.P.; Ip, Y.K. Ventricle and outflow tract of the African lungfish Protopterus dolloi. J. Morphol. 2005, 265, 43–51.
- Putnam, J.L.; Parkerson, J.B. Anatomy of the heart of the Amphibia II. Cryptobranchus alleganiensis. Herpetologica 1985, 41, 287–298.
- Davies, F.; Francis, E.T.B. The heart of the salamander (Salamandra salamandra, L.), with special reference to the conducting (connecting) system and its bearing on the phylogeny of the conducting systems of avian and mammalian hearts. Philos. Trans. R. Soc. Lond. 1941, 231, 99–130.
- Morris, R.W. Function of the anuran conus arteriosus. J. Exp. Biol. 1974, 61, 503–520.
- Lawson, R. The comparative anatomy of the circulatory system. In Hyman’s Comparative Anatomy; Wake, M.H., Ed.; University of Chicago Press: Chicago, IL, USA, 1979; pp. 448–554.
- Kashyap, H.V. The structure of the heart of Typhlops (Reptilia: Ophidia). J. Zool. Soc. India 1950, 2, 42–49.
- Webb, G.J.W.; Heatwole, H.; De Bavay, J. Comparative cardiac anatomy of the Reptilia: II. A critique of the literature on the Squamata and Rhynchocephalia. J. Morphol. 1974, 142, 1–20.
- Webb, G.J.W. Comparative cardiac anatomy of the Reptilia: III. The heart of crocodilians and hypothesis on the completion of the interventricular septum of crocodilians and birds. J. Morphol. 1979, 161, 221–240.
- Young, B.A.; Lillywhite, H.B.; Wassersug, R.J. On the structure of the aortic valves in snakes (Reptilia: Serpentes). J. Morphol. 1993, 216, 141–159.
- López, D.; Durán, A.C.; Sans-Coma, V. Formation of cartilage in semilunar valves of chick and quail. Ann. Anat. 2000, 182, 349–359.
- Bartyzel, B.J.; Charuta, A.; Barszcz, K.; Kolesnik, A.; Kobryn, H. Morphology of the aortic valve of Gallus gallus f. domestica. Bull. Vet. Inst. Pulawy 2009, 53, 147–151.
- Tadjalli, M.; Ghazi, S.R.; Parto, P. Gross anatomy of the heart in ostrich (Struthio camelus). Iran. J. Vet. Res. Schiraz Univ. 2009, 10, 21–27.
- Thubrikar, M.; Piepgrass, W.C.; Shaner, T.W.; Nolan, S.P. The design of the normal aortic valve. Am. J. Physiol. 1981, 241, H795–H801.
- Bierbach, B.O.; Aicher, D.; Issa, O.A.; Bomberg, H.; Glombitza, P.; Schäfers, H.J. Aortic root configuration determine aortic valve function. Eur. J. Cardiothorac. Surg. 2010, 38, 400–406.
- Rankin, J.S.; Bone, M.C.; Fries, P.M.; Aicher, D.; Schäfers, H.J.; Crooke, P.S. A refined hemispheric model of normal aortic valve root geometry. J. Thorac. Cardiovasc. Surg. 2013, 146, 103–108.
- Fedak, P.W. Bicuspid aortic valve syndrome: Heterogeneous but predictable? Eur. Heart J. 2008, 29, 432–433.
- Siu, S.C.; Silversides, C.K. Bicuspid aortic valve disease. J. Am. Coll. Cardiol. 2010, 55, 2789–2800.
- Roberts, W.C. The congenitally bicuspid aortic valve. A study of 85 autopsy cases. Am. J. Cardiol. 1970, 26, 72–83.
- Ward, C. Clinical significance of the bicuspid aortic valve. Heart 2000, 83, 81–85.
- Basso, C.; Boschello, M.; Perrone, C.; Mecenero, A.; Cera, A.; Bicego, D.; Thiene, G.; de Dominicis, E. An echocardiographic survey of primary school children for bicuspid aortic valve. Am. J. Cardiol. 2004, 93, 661–663.
