Aegerolysins are remarkable proteins. They are distributed over the tree of life, being relatively widespread in fungi and bacteria, but also present in some insects, plants, protozoa, and viruses. Their function, in particular, is intriguing. Aegerolysin proteins are involved in various interactions by recognizing a molecular receptor in the target organism. Despite their abundance in cells of certain developmental stages and their presence in secretomes, only a few aegerolysins have been studied in detail. Formation of pores with various larger non-aegerolysin-like protein partners is one of the possible responses of the aegerolysin-producing organism in competitive exclusion of other organisms from the ecological niche.
- aegerolysins
- bacteria
- fungi
- insecticidal
- lipid binding
- lifestyle
- membrane-attack complex/perforin domain (MACPF)
- pore forming proteins
1. Introduction
2. Aegerolysins
| Organism Name | Other Names | Taxonomy | Lifestyle/Niche | Reference |
|---|---|---|---|---|
| Fungi | ||||
| Pleurotus ostreatus | Oyster mushroom Hiratake |
Agaricomycotina Agaricales |
Saprotroph White rot Nematocidal |
[15] |
| Pleurotus eryngii | King oyster or trumpet or brown mushroom Boletus of the steppes French horn mushroom Aliʻi oyster |
Agaricomycotina Agaricales |
Saprotroph Grassland-litter decomposer Facultatively biotrophic Nematocidal |
[16] |
| Cyclocybe aegerita | Agrocybe aegerita Poplar mushroom Tea tree mushroom Cha shu gu Yanagi-matsutake Sword-belt mushroom Velvet pioppini |
Agaricomycotina Agaricales |
Saprotroph Weak white rot on hardwoods Facultatively pathogenic |
[17] |
| Moniliophthora perniciosa | Crinipellis perniciosa Witches’ broom disease |
Agaricomycotina Agaricales |
Hemibiotrophic plant pathogen Broad range of host |
[18] |
| Lignosus rhinocerotis | Tiger milk mushroom | Agaricomycotina Polyporales |
Saprotroph White rot |
[19] |
| Neosartorya fumigata | Aspergillus fumigatus | Eurotimycetes Eurotiales |
Saprotroph Ubiquitous in soil and compost Human (opportunistic) pathogen |
[20,21,22,23] |
| Aspergillus niger | Eurotimycetes Eurotiales |
Saprotroph Ubiquitous in soil and compost Human opportunistic pathogen |
[20,21,24,25,26,27] | |
| Aspergillus terreus | Eurotimycetes Eurotiales |
Saprotroph Human opportunistic pathogen |
[20,21,28] | |
| Aspergillus oryzae | Eurotimycetes Eurotiales |
Saprotroph | [20,21,29] | |
| Beauveria bassiana | Sordariomycetes Hypocreales |
Entomopathogen Endophyte Soil and insects |
[30,31] | |
| Hypocrea atroviridis | Trichoderma atroviride | Sordariomycetes Hypocreales |
Mycoparasitic (including oomycetes) Cosmopolitan, soil |
[32,33,34,35] |
| Alternaria geisen | Black spot of Japanese pear | Dothideomycetes Pleosporales |
Plant pathogen | [36,37] |
| Bacteria | ||||
| Bacillus thuringiensis | Firmicutes | Ubiquitous opportunistic pathogen on vertebrates, plants, insects, nematodes, mollusks, protozoan, animal, and human parasites. Soils, grain dusts, dead insects, water Aerobic and spore-forming |
[38,39] | |
| Paraclostridium bifermentans subsp. malaysia |
Clostridium bifermentans subsp. malaysia |
Firmicutes | Anaerobic, forming endospores Mosquito larvicidal |
[40] |
| Alcaligenes faecalis | Proteobacteria | Soil, water, environments associated with humans Human opportunistic pathogen Nematocidal |
[41] | |
| Pseudomonas aeruginosa | Proteobacteria | Ubiquitous opportunistic pathogen on: humans, vertebrates, plants, and insects |
[42,43,44,45,46] | |
| Insecta | ||||
| Chrysodeixis includens | Pseudoplusia includes | Lepidoptera Noctuidae |
Plant pest (defoliator) Larvae feed on a wide range of plants |
[47,48] |
| Viria | ||||
| Trichoplusia ni ascovirus 6a1 | Trichoplusia ni ascovirus 2c | Varidnaviria Ascoviridae |
Obligate pathogen Pseudoplusia includens moth larvae |
[47,49,50,51] |
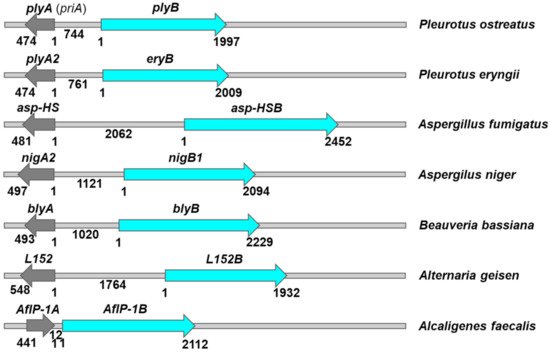
3. Aegerolysin Binary Partner Proteins
| Short Name | Aegerolysin/ Structure |
Membrane Receptor |
Function | Partner Protein Short Name | Partner Protein/ Structure |
Organism | Reference |
|---|---|---|---|---|---|---|---|
| PlyA | Pleurotolysin A PDB ID: 4OEBA |
SM/Chol | n.d. | PlyB | Pleurotolysin B PDB ID: 4OEJ Membrane embedded PlyA/PlyB pore PDB ID: 4V2T |
Pleurotus ostreatus | [59,61] |
| OlyA | Ostreolysin A | SM/Chol CPE/Chol Lipid rafts |
Involvement in mushroom fruiting Anticancer (+PlyB) |
PlyB | Pleurotolysin B PDB ID: 4OEJ |
Pleurotus ostreatus | [63,64,65,66,67,68,69,70,71,76,80,84,85,86,102,104] |
| OlyA6 | Ostreolysin A6 PDB ID: 6MYJ |
SM/Chol CPE/Chol CAEP/POPC/Chol Lipid rafts |
Insecticidal (+PlyB) | PlyB | Pleurotolysin B PDB ID: 4OEJ |
Pleurotus ostreatus | [72,73,74,75,78,79,80,81,82,83,87] |
| rOly | Recombinant ostreolysin | Lipid rafts? | Antiproliferative Pro-apoptotic |
n.d. | n.d. | Pleurotus ostreatus | [88,89,90] |
| PlyA2 | Pe.PlyA/Pleurotolysin A2 | SM/Chol CPE/Chol CPE Lipid rafts |
Insecticidal (+PlyB) |
EryB | Erylysin B | Pleurotus eryngii | [15,79,80,93,94,95,96] |
| EryA | Erylysin A | CPE/Chol CL/DPPC/Chol |
Insecticidal (+PlyB) Inhibition of cytokinesis |
No | No | Pleurotus eryngii | [78,80,96,98,99] |
| Aa-Pri1 | Aegerolysin Aa-Pri1 | n.d. | No | No | Agrocybe aegerita | [63,100] | |
| MpPRIA1 | Putative aegerolysin | n.d. | MpPLYB? | n.d. | Moniliophthora perniciosa | [101] | |
| MpPRIA2 | Putative aegerolysin | n.d. | MpPLYB? | n.d. | Moniliophthora perniciosa | [101] | |
| GME7309 | GME7309_g aegerolysin-domain-containing protein | n.d. | n.d. | n.d. | Lignosus rhinocerotis | [105] | |
| Asp-HS | Asp-hemolysin | Oxidized low-density lipoproteins | Cytotoxic effects on murine macrophages and vascular endothelial cells Induce cytokine genes |
Asp-HSB | n.d. | Aspergillus fumigatus | [107,108,109,110,111,112,113,114,115] |
| Asp-HS-like | Asp hemolysin-like | n.d. | n.d. | No | No | Aspergillus fumigatus |
[114] |
| NigA1 | Nigerolysin A1 | n.d. | n.d. | No | No | Aspergillus niger | [91,92] |
| NigA2 | Nigerolysin A2 | CPE/Chol | n.d. | NigB1 | Nigerolysin B1 | Aspergillus niger | [91,92] |
| Ter | Terrelysin | n.d. | n.d. | No | No | Aspergillus terreus | [28,116,117] |
| AoHlyA | Aspergillus oryzae hemolysin | n.d. | n.d. | No | No | Aspergillus oryzae | [118,119,120] |
| BlyA | Beauveriolysin A | SM/Chol CPE/Chol |
n.d. | BlyB | Beauveriolysin B | Beauveria bassiana | [52] |
| Agl1 | Trichoderma atroviride aegerolysin | Conidiation Antagonism |
n.d. | No | No | Trichoderma atroviride | [128] |
| L152 | Alternaria geisen aegerolysin | n.d. | n.d. | L152B | n.d. | Alternaria geisen | [36] |
| Cry34Ab1 (Gpp34Ab1) |
13.6 kDa Insecticidal crystal protein PDB ID: 4JOX |
Unknown protein receptor | Insecticidal (+Cry34Ab1) |
Cry35Ab1 (Tpp35Ab1) |
43.8 kDa insecticidal crystal protein PDB ID: 4JP0 |
Bacillus thuringiensis | [39,129,130,131,132,133,134,135,136] |
| Cbm17.1 | Hemolysin-like protein Cbm17.1 | n.d. | Insecticidal (+Cry16Aa/Cry17Aa/ Cbm17.2) |
Cry16Aa, Cry17Aa, Cbm17.2 | Pesticidal crystal-like protein Cry16Aa and Cry17Aa, Hemolysin-like protein Cbm17.2 | Clostridium bifermentans | [40,140,142] |
| Cbm17.2 | Hemolysin-like protein Cbm17.2 | n.d. | Insecticidal (+Cry16Aa/Cry17Aa/ Cbm17.1) |
Cry16Aa, Cry17Aa, Cbm17.2 | Pesticidal crystal-like protein Cry16Aa and Cry17Aa, Hemolysin-like protein Cbm17.1 | Clostridium bifermentans | [40,140,142] |
| AfIP-1A | Two-component insecticidal protein 16 kDa unit PDB ID: 5V3S |
Unknown protein receptor AfIP-1A/AfIP-1B membrane pore |
n.d. | AfIP-1B | Two-component insecticidal protein 77 kDa unit |
Alcaligenes faecalis | [143,144] |
| RahU | RahU protein PDB ID: 6ZC1 |
CPE/Chol | n.d. | No | No | Pseudomonas aeruginosa | [145,148] |
| P23 | n.d. | n.d. | n.d. | No | No | Pseudoplusia includes | [48] |
| TnAV2cgp029 | n.d. | n.d. | n.d. | No | No | Trichoplusia ni ascovirus 2c | [49,50,149,150,152] |
This entry is adapted from the peer-reviewed paper 10.3390/toxins14090629
