Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The prokineticin system (PROK) consists of the prokineticin 1 (PROK1) and prokineticin 2 (PROK2) proteins. Through the activation of two G-protein receptors (PROKR1 and PROKR2) regulate a wide range of biological functions, including gastrointestinal motility, circadian rhythm regulation, neurogenesis, angiogenesis, pain perception, and mood regulation. Recently, new evidence confirms and extends the knowledge on the role of the PROK2 system in the male reproductive system, opening new scenarios in the field of male infertility
- male infertility
- PROK system
- PROK2
1. Prokineticin System
The prokineticin (PROK) system consists of the prokineticin 1 (PROK1) and prokineticin 2 (PROK2) proteins, identified as the mammalian homologs of two amphibious proteins, the intestinal toxin mamba (MIT-1) and Bv8, respectively. PROKs regulate a wide range of biological functions through the activation of two G-protein receptors: PROKR1 and PROKR2 [1][2]. PROK signaling has been involved in several physiological functions, including gastrointestinal motility [3][4], thus accounting for their family name “prokineticins”, circadian rhythm regulation [5][6][7], neurogenesis [8][9], angiogenesis [10][11][12], pain perception [12][13], mood regulation [14][15][16]. Dysregulation of PROK signaling has been observed in different pathological conditions, such as cancer, ischemia, and neurodegeneration, in which PROK system seems to be a promising therapeutic target (see Figure 1).
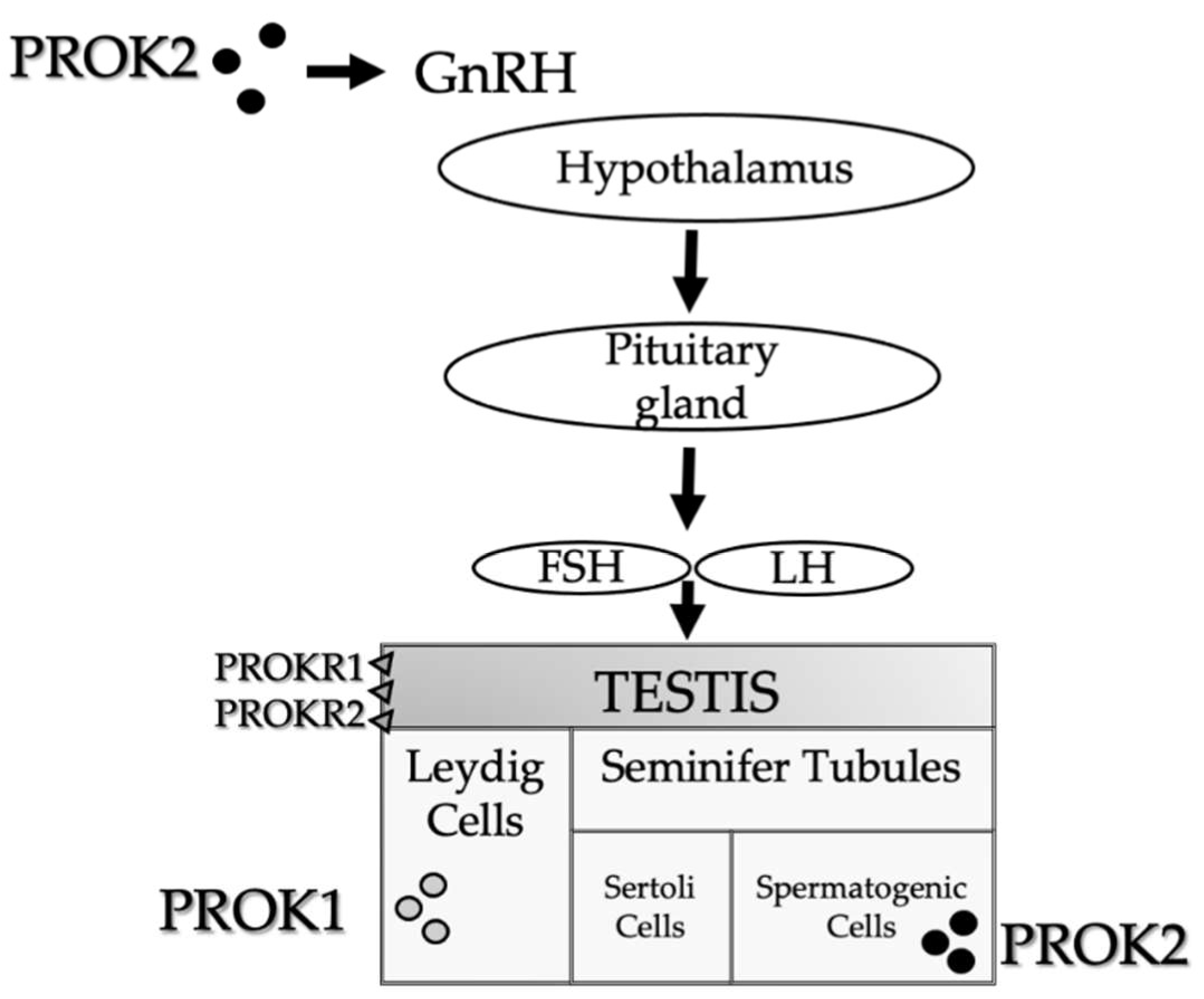
Figure 1. PROKs schematic representation in the male reproductive system. PROK1 is mainly expressed in steroidogenic organs, particularly in the Leydig cells. PROK2 is primarily expressed in the central nervous system, which influences the olfactory bulb development and GnRH neural migration, and in non-steroidogenic cells of the testes. PROK2 expression is limited to the seminiferous tubules in the primary spermatocytes. In the testes, both receptors (PROKR1 and PROKR2) are expressed in endothelial cells in the interstitial tissue.
Among the numerous biological functions modulated by the PROK system, the role of the development and maturation of the reproductive organs in humans is particularly significant. In general, PROK ligands are considered angiogenic and mitogenic/survival factors, involved in the high rate of endothelial cell turnover and also in the testis [17]. PROK1 is predominantly expressed in steroidogenic organs (ovary, testis, adrenal cortex, placenta), and it has been described to have regulatory properties on the gonads [18][19][20]. In males, PROK1 is abundantly expressed in the testes during embryonal testicular development. Finally, in adult men, PROK1 is expressed in Leydig cells (102),
From the anatomical point of view, PROK2 is predominantly expressed in the central nervous system and non-steroidogenic cells of the testes [7]. Concerning human reproduction, PROK2 plays a major role in olfactory bulb development and GnRH neural migration. In both humans and mice, PROK2 expression is restricted to the seminiferous tubules in the primary spermatocytes [21]. In the testes, both receptors are expressed on endothelial cells in the interstitial tissue [17].
2. Prokineticin 2 in Male Reproduction: Physiology and Pathology
2.1. Pre-Clinical Studies
2.1.1. Varicocele
The potential role of PROK2 in experimental varicocele-induced rat testes has been suggested by Tu et al., and demonstrated a significantly increased expression of PROK2 mRNA in the testis compared to the control group. This work was born by evidence of the involvement of PROK2 in endothelial proliferation and in angiogenesis, both processes particularly critical in the testis. The researchers demonstrated that the PROK2 system is involved in the hypoxia-induced testicular injury, hypothesizing, on the one hand, a protective role of PROK2, inducing endothelial proliferation, probably in an attempt to protect germ cells from apoptosis. On the other hand, as a chemokine, PROK2 supported a pro-inflammatory environment, favoring male infertility in the experimental model [22].
A more recent study evaluated the mechanisms affecting spermatogenesis in the presence of varicocele. The researchers revealed that the varicocele-induced oxidative stress increased the expression of the PROK2, leading to apoptosis of spermatocytes. The same researchers demonstrated that in vitro spermatocyte-derived cell line cultured in the presence of H2O2, to mimic the oxidative stress state of varicocele, overexpressed both PROK2 mRNA and protein, confirming the modulation of the PROK2 system in this oxidative stress-associated pathological condition [23].
Based on these previous studies, a recent experimental work evaluated the mechanism underlying the positive effect of lycopene, a natural extract with antioxidative and anti-inflammatory properties, on hypoxia-induced testicular injury in rats. It was found that lycopene inhibited the PROK2 expression, and consequently the secretion function and spermatogenic function recovered in the testis [24].
2.1.2. Orchitis
It has been proven that the pathogenesis of orchitis mainly includes inflammatory cytokine imbalance, oxidative stress, apoptosis, and the PROK2 pathway. In lipopolysaccharide-induced acute orchitis, administration of methane, an interesting formulation with beneficial properties [25], decreased pro-inflammatory mediators, and over-expressed the anti-inflammatory interleukin IL-10 in the rat testes [26]. In particular, it was found that methane significantly prolonged rat survival, improved the testis condition, alleviated lipopolysaccharide-induced histological changes, and reduced apoptotic cells in the testes [26]. Furthermore, methane significantly increased superoxide dismutase, decreased malondialdehyde, and reduced testicular expression of PROK2 and PROKR1. Therefore, methane exerts therapeutic effects on acute orchitis and might be a new and convenient strategy for the treatment of inflammation-related testicular diseases. [26]. Indeed, methane, the simplest organic compound, was deemed to have little physiological action for decades. However, recently, many basic studies have discovered that methane has several important biological effects that can protect cells and organs from inflammation, oxidant, and apoptosis [25].
Recently, the NLRP3 (NLR family pyrin domain containing 3), the molecular sensor of the NLRP3 inflammasome, has been identified in mouse, human and non-human primates (marmoset and rhesus macaque) testes. Sertoli cells of all species expressed NLRP3, and the expression preceded puberty. In addition, peritubular cells of the adult human testes expressed NLRP3. NLRP3 and associated genes (PYCARD, CASP1, IL1B) were also found in isolated human testicular peritubular cells and the mouse Sertoli cell line TM4. Due to the involvement of inflammatory events in male infertility, by using a mouse model of male infertility, a group of researchers identified NLRP3 as a novel player in testicular immune regulation because of its expression in the somatic cells of the testis involved in testicular immune surveillance [27]. Activation of the NLRP3 inflammasome in orchitis promotes the secretion and maturation of IL-1β and, thus, decreases male fertility. Su and co-workers developed a uropathogenic Escherichia coli (UPEC) rat orchitis model, through which they investigated the NLRP3 inflammatory pathway proteins in testicular macrophages, and in particular, the expression of CaSR (calcium-sensing receptor), responsible for the NLRP3 activation. Interestingly, the researchers found that UPEC infection induced large amounts of PROK2 secreted into the cytoplasm to stimulate the activation of CaSR and activate the NLRP3 inflammasome by increasing the level of calcium ions in the cytoplasm of macrophages. This research puts on evidence a regulatory role of the PROK2 in promoting the NLRP3 pathway [28].
In summary, the most recent pre-clinical studies related to the varicocele and orchitis models disclosed an overexpression of PROK2, a potential new anti-inflammatory therapeutic target for male infertility (see Figure 2).
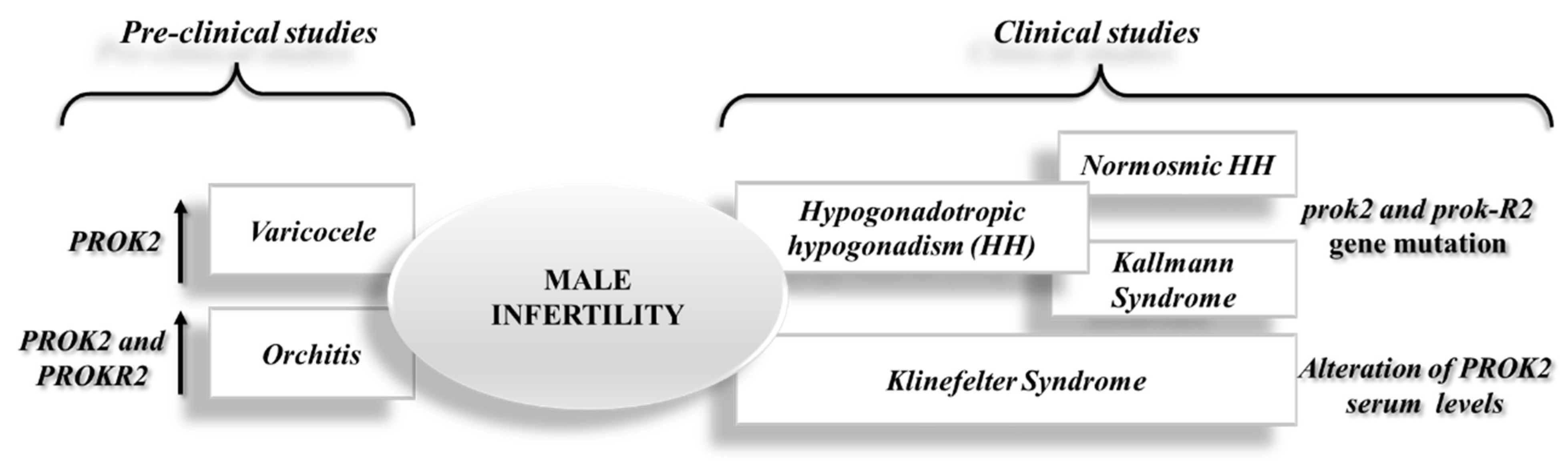
Figure 2. Schematic representation of recent advance on the role of the PROK2 system in male infertility from pre-clinical and clinical studies.
2.2. Clinical Studies
2.2.1. Hypogonadotropic Hypogonadism
As previously said, HH is a disease caused by insufficient stimulation by the luteinizing hormone (LH) and follicle-stimulating hormone (FSH) of otherwise normal functioning gonads. HH can be congenital or acquired, isolated or combined with other secretory defects of the pituitary hormones. The isolated HH can be associated with a normal or altered sense of smell, identifying, respectively, the normosmic HH (nHH) or the Kallmann syndrome. The clinical picture related to HH varies according to the age of onset and can be associated with extra-reproductive clinical manifestations. Kallmann syndrome was originally thought to be caused by mutations in a specific gene located on the X chromosome, KAL1, but it was soon discovered that this genetic defect was present in a minority of patients [29]. Therefore, since the causal event of HH was missing, the classification of “idiopathic” HH (IHH) was used. The observation of family cases with variable modes of inheritance (X-linked or autosomal dominant/recessive) indicated that IHH has a strong genetic component at its base, albeit heterogeneous. In the last decade, contributions from cellular and animal models, together with genetic studies on affected patients, have made it possible to discover new genetic determinants of IHH (both nIHH and Kallmann syndrome) and to better understand their pathophysiology. To date, the identified genes are involved in the development/migration or activation of secreting GnRH neurons, in the synthesis/secretion of GnRH, or in the mediation of the action of GnRH at the pituitary level.
2.2.2. Klinefelter Syndrome
Klinefelter syndrome refers to a group of male chromosomal diseases linked to the presence of at least one supernumerary X chromosome compared to the normal male karyotype 46, XY. Although they are often considered as a single nosological entity, this condition should be differentiated from higher-grade aneuploidies (HGAs), in which there is more than one additional X or Y chromosome, which have different clinical, hormonal and metabolic manifestations [30].
Klinefelter syndrome is the most frequent genetic form of male hypogonadism. Klinefelter syndrome symptoms are highly variable and can include late or incomplete puberty, testicular atrophy and low production of testosterone, poor development of facial and body hair, gynecomastia, infertility or reduced fertility, and muscle weakness. Klinefelter patients may also be characterized by other important clinical aspects, including a tendency to develop visceral obesity, dyslipidemia, hypertension, and diabetes mellitus, and hence metabolic syndrome, increasing the cardiovascular risk [31]. In some cases, albeit rarely, there are also delays in language development and reading difficulties (dyslexia). Concerning the last point, researchers' recent study conducted in adolescents affected by Klinefelter syndrome showed reduced brain-derived neurotrophic factor/BDNF serum levels, associated with a decrease in inflammatory markers, disclosing a disrupted immune system and neurotrophins pathways in this pathological condition [32].
This entry is adapted from the peer-reviewed paper 10.3390/biomedicines10102389
References
- Negri, L.; Lattanzi, R.; Giannini, E.; Melchiorri, P. Bv8/Prokineticin proteins and their receptors. Life Sci. 2007, 81, 1103–1116.
- Zhou, Q.Y.; Meidan, R. Biological function of prokineticins. Results Probl. Cell Differ. 2008, 46, 181–199.
- Li, M.; Bullock, C.M.; Knauer, D.J.; Ehlert, F.J.; Zhou, Q.Y. Identification of two prokineticin cDNAs: Recombinant proteins potently contract gastrointestinal smooth muscle. Mol. Pharmacol. 2001, 59, 692–698.
- Wade, P.R.; Palmer, J.M.; Mabus, J.; Saunders, P.R.; Prouty, S.; Chevalier, K.; Gareau, M.G.; McKenney, S.; Hornby, P.J. Prokineticin-1 evokes secretory and contractile activity in rat small intestine. Neurogastroenterol. Motil. 2010, 22, e152–e161.
- Zhou, Q.Y.; Cheng, M.Y. Prokineticin 2 and circadian clock output. FEBS J. 2005, 272, 5703–5709.
- Cheng, M.Y.; Bullock, C.M.; Li, C.; Lee, A.G.; Bermak, J.C.; Belluzzi, J.; Weaver, D.R.; Leslie, F.M.; Zhou, Q.Y. Prokineticin 2 transmits the behavioural circadian rhythm of the suprachiasmatic nucleus. Nature 2002, 417, 405–410.
- Cheng, M.Y.; Bittman, E.L.; Hattar, S.; Zhou, Q.Y. Regulation of prokineticin 2 expression by light and the circadian clock. BMC Neurosci. 2005, 6, 17.
- Ng, K.L.; Li, J.D.; Cheng, M.Y.; Leslie, F.M.; Lee, A.C.; Zhou, Q.Y. Neuroscience: Dependence of olfactory bulb neurogenesis on prokineticin 2 signaling. Science 2005, 308, 1923–1927.
- Zhang, C.; Ng, K.L.; Li, J.D.; He, F.; Anderson, D.J.; Sun, Y.E.; Zhou, Q.Y. Prokineticin 2 is a target gene of proneural basic helix-loop-helix factors for olfactory bulb neurogenesis. J. Biol. Chem. 2007, 282, 6917–6921.
- Meng, S.; Gu, Q.; Yang, X.; Lv, J.; Owusu, I.; Matrone, G.; Chen, K.; Cooke, J.P.; Fang, L. TBX20 regulates angiogenesis through the prokineticin 2-prokineticin receptor 1 pathway. Circulation 2018, 138, 913–928.
- Monnier, J.; Samson, M. Prokineticins in angiogenesis and cancer. Cancer Lett. 2010, 296, 144–149.
- Kurebayashi, H.; Goi, T.; Shimada, M.; Tagai, N.; Naruse, T.; Nakazawa, T.; Kimura, Y.; Hirono, Y.; Yamaguchi, A. Prokineticin 2 (PROK2) is an important factor for angiogenesis in colorectal cancer. Oncotarget 2015, 6, 26242–26251.
- Liu, B.; Qiao, L.; Liu, K.; Liu, J.; Piccinni-Ash, T.J.; Chen, Z.F. Molecular and neural basis of pleasant touch sensation. Science 2022, 376, 483–491.
- Amodeo, G.; Verduci, B.; Sartori, P.; Procacci, P.; Conte, V.; Balboni, G.; Sacerdote, P.; Franchi, S. The antagonism of the prokineticin system counteracts bortezomib induced side effects: Focus on mood alterations. Int. J. Mol. Sci. 2021, 22, 10256.
- Kishi, T.; Kitajima, T.; Tsunoka, T.; Okumura, T.; Ikeda, M.; Okochi, T.; Kinoshita, Y.; Kawashima, K.; Yamanouchi, Y.; Ozaki, N.; et al. Possible association of prokineticin 2 receptor gene (PROKR2) with mood disorders in the Japanese population. NeuroMolecular Med. 2009, 11, 114–122.
- Moschetti, G.; Amodeo, G.; Paladini, M.S.; Molteni, R.; Balboni, G.; Panerai, A.; Sacerdote, P.; Franchi, S. Prokineticin 2 promotes and sustains neuroinflammation in vincristine treated mice: Focus on pain and emotional like behavior. Brain. Behav. Immun. 2019, 82, 422–431.
- Ferrara, N.; LeCouter, J.; Lin, R.; Peale, F. EG-VEGF and Bv8: A novel family of tissue-restricted angiogenic factors. Biochim. Biophys. Acta Rev. Cancer 2004, 1654, 69–78.
- Martin, C.; Balasubramanian, R.; Dwyer, A.A.; Au, M.G.; Sidis, Y.; Kaiser, U.B.; Seminara, S.B.; Pitteloud, N.; Zhou, Q.-Y.; Crowley, W.F.J. The role of the prokineticin 2 pathway in human reproduction: Evidence from the study of human and murine gene mutations. Endocr. Rev. 2011, 32, 225–246.
- Maldonado-Perez, D.; Evans, I.; Denison, F.; Millar, R.P.; Jabbour, H.N. Potential roles of the prokineticins in reproduction. TRENDS Endocrinol. Metab. 2007, 18, 66–72.
- LeCouter, J.; Lin, R.; Tejada, M.; Frantz, G.; Peale, F.; Hillan, K.J.; Ferrara, N. The endocrine-gland-derived VEGF homologue Bv8 promotes angiogenesis in the testis: Localization of Bv8 receptors to endothelial cells. Proc. Natl. Acad. Sci. USA 2003, 100, 2685–2690.
- Wechselberger, C.; Puglisi, R.; Engel, E.; Lepperdinger, G.; Boitani, C.; Kreil, G. The mammalian homologues of frog Bv8 are mainly expressed in spermatocytes. FEBS Lett. 1999, 462, 177–181.
- Baazeem, A.; Belzile, E.; Ciampi, A.; Dohle, G.; Jarvi, K.; Salonia, A.; Weidner, W.; Zini, A. Varicocele and male factor infertility treatment: A new meta-analysis and review of the role of varicocele repair. Eur. Urol. 2011, 60, 796–808.
- Li, Y.; Zhou, T.; Su, Y.F.; Hu, Z.Y.; Wei, J.J.; Wang, W.; Liu, C.Y.; Zhao, K.; Zhang, H.P. Prokineticin 2 overexpression induces spermatocyte apoptosis in varicocele in rats. Asian J. Androl. 2020, 22, 500–506.
- Wang, H.; Zhu, B.; Yu, L.; Li, Q.; Li, S.; Wang, P.; Jing, T.; Men, T. Lycopene Attenuates Hypoxia-Induced Testicular Injury by Inhibiting PROK2 Expression and Activating PI3K/AKT/mTOR Pathway in a Varicocele Adult Rat. Evid.-Based Complement. Altern. Med. 2021, 2021, 3471356.
- Jia, Y.; Li, Z.; Liu, C.; Zhang, J. Methane Medicine: A Rising Star Gas with Powerful Anti-Inflammation, Antioxidant, and Antiapoptosis Properties. Oxid. Med. Cell. Longev. 2018, 2018, 1912746.
- Huang, C.; Zhang, W.; Sun, A.; Zhang, X.; Guo, J.; Ji, R.; Qiao, L.; Sun, X.; Zhao, D. Methane Ameliorates Lipopolysaccharide-Induced Acute Orchitis by Anti-inflammatory, Antioxidative, and Antiapoptotic Effects via Regulation of the PK2/PKR1 Pathway. Oxid. Med. Cell. Longev. 2020, 2020, 7075836.
- Walenta, L.; Schmid, N.; Ullrich Schwarzer, J.; Köhn, F.M.; Urbanski, H.F.; Behr, R.; Strauss, L.; Poutanen, M.; Mayerhofer, A. NLRP3 in somatic non-immune cells of rodent and primate testes. Reproduction 2018, 156, 231–238.
- Su, Y.; Zhang, Y.; Hu, Z.; He, L.; Wang, W.; Xu, J.; Fan, Z.; Liu, C.; Zhang, H.; Zhao, K. Prokineticin 2 via Calcium-Sensing Receptor Activated NLRP3 Inflammasome Pathway in the Testicular Macrophages of Uropathogenic Escherichia coli-Induced Orchitis. Front. Immunol. 2020, 11, 570872.
- Bonomi, M.; Libri, D.V.; Guizzardi, F.; Guarducci, E.; Maiolo, E.; Pignatti, E.; Asci, R.; Persani, L. New understandings of the genetic basis of isolated idiopathic central hypogonadism. Asian J. Androl. 2012, 14, 49–56.
- Spaziani, M.; Mileno, B.; Rossi, F.; Granato, S.; Tahani, N.; Anzuini, A.; Lenzi, A.; Radicioni, A.F. Endocrine and metabolic evaluation of classic Klinefelter syndrome and high-grade aneuploidies of sexual chromosomes with male phenotype: Are they different clinical conditions? Eur. J. Endocrinol. 2018, 178, 343–352.
- Spaziani, M.; Radicioni, A.F. Metabolic and cardiovascular risk factors in Klinefelter syndrome. Am. J. Med. Genet. Part C Semin. Med. Genet. 2020, 184, 334–343.
- Tarani, L.; Ceci, F.M.; Carito, V.; Ferraguti, G.; Petrella, C.; Greco, A.; Ralli, M.; Minni, A.; Spaziani, M.; Isidori, A.M.; et al. Neuroimmune Dysregulation in Prepubertal and Adolescent Individuals Affected by Klinefelter Syndrome. Endocrine, Metab. Immune Disord. Drug Targets, 2022; online ahead of print.
This entry is offline, you can click here to edit this entry!
