Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The blood–cerebrospinal fluid (CSF) barrier (BCSFB), an under-studied brain barrier site compared to the blood–brain barrier (BBB), can be considered a potential therapeutic target to improve the delivery of CNS therapeutics and provide brain protection measures. Therefore, leveraging robust and authentic in vitro models of the BCSFB can diminish the time and effort spent on unproductive or redundant development activities by a preliminary assessment of the desired physiochemical behavior of an agent toward this barrier.
- BCSFB
- blood–cerebrospinal fluid barrier
- in vitro model
1. Background
The research and development platforms of therapeutics targeted to the brain or aimed to be excluded from the central nervous system (CNS) depends mainly on their accomplishment in assessing the reciprocal behavior of brain barriers and the pharmacologically active agent. This effort is made to conquer the economic and technical burden on the pharmaceutical industry attributable to high risk and poor approval rates of neurotherapeutics. During the long journey of drug development, based on the nature of the product under study and in the cases of pregnant women, neonates, and any conditions with the possibility that drugs might have short- or long-term damaging effects, substantial data are required to prove that a substance is excluded effectively from the brain. On the other hand, when the aim is efficient drug delivery to the CNS, the development of neurotherapeutics faces a number of major challenges, with difficulty in delivering them to their desired site of action in the brain being on top. The presence of highly selective barriers (the blood–brain barrier (BBB) and the blood–cerebrospinal fluid (CSF) barrier (BCSFB)) and the complexity of the highly regulated brain environment are among the factors responsible for the elevated attrition percentage during the development of these CNS-targeting medicines.
The BBB and BCSFB are of utmost importance to pharmaceutical drug discovery, as both barriers provide obstacles to the penetration and delivery of therapeutics and diagnostics designed for the management of CNS complications and disorders. Presently, leading research has been concentrated on the brain vascular endothelium as a medicinal target to improve the delivery of brain therapeutics. However, the more poorly noticed BCSFB is also an essential gateway to the CNS, since it is broadly accepted that most substances, even macromolecules, that successfully reach the CSF, can find their way further to the brain parenchyma. Taken together with its considerable surface area, which is estimated to be ~25 to 50% the size of the inner capillary surface area of the brain [1], the BCSFB can be regarded as a target for the CNS delivery of (neuro)therapeutics [2][3]. Notably, the unique role of this barrier to regulate the composition of the CSF offers the BCSFB also the privilege of augmenting the levels of certain compounds and drug candidates in the CSF to accomplish therapeutic benefits and availability to the brain parenchyma.
Species relevant in vitro model systems of the BCSFB play an essential role in enabling the understanding of this barrier’s properties and developing potential interventional techniques and therapeutics. In the pharmaceutical, pharmacological, and toxicological fields, these models harbor the potential to be applied for investigational drugs/compounds screenings, permeability and transport assays, (neuro)toxicological evaluations, and mechanistic molecular pharmacological studies and related assays at the pre-clinical level. Due to many economic concerns, ethical issues, and the necessity to comply with the three R rules (the replacement, reduction, and refinement of animal subject experiments) and bypassing animal testing as far as possible, there has been a great effort in past decades toward establishing robust and convenient models to study basic characteristics of the barriers and also to facilitate the research and pre-clinical investigations of neurotherapeutics in the laboratory under controlled conditions. Such models should authentically mimic the in vivo microenvironment phenotype of the BCSFB and demonstrate representative properties of functional elements of tight junctions (TJs) leading to high transepithelial electrical resistance (TEER), restricted paracellular permeability, low non-specific pinocytic activity, and the expression of receptors and transporters.
2. Structure and Physio-Anatomical Features of the BCSFB
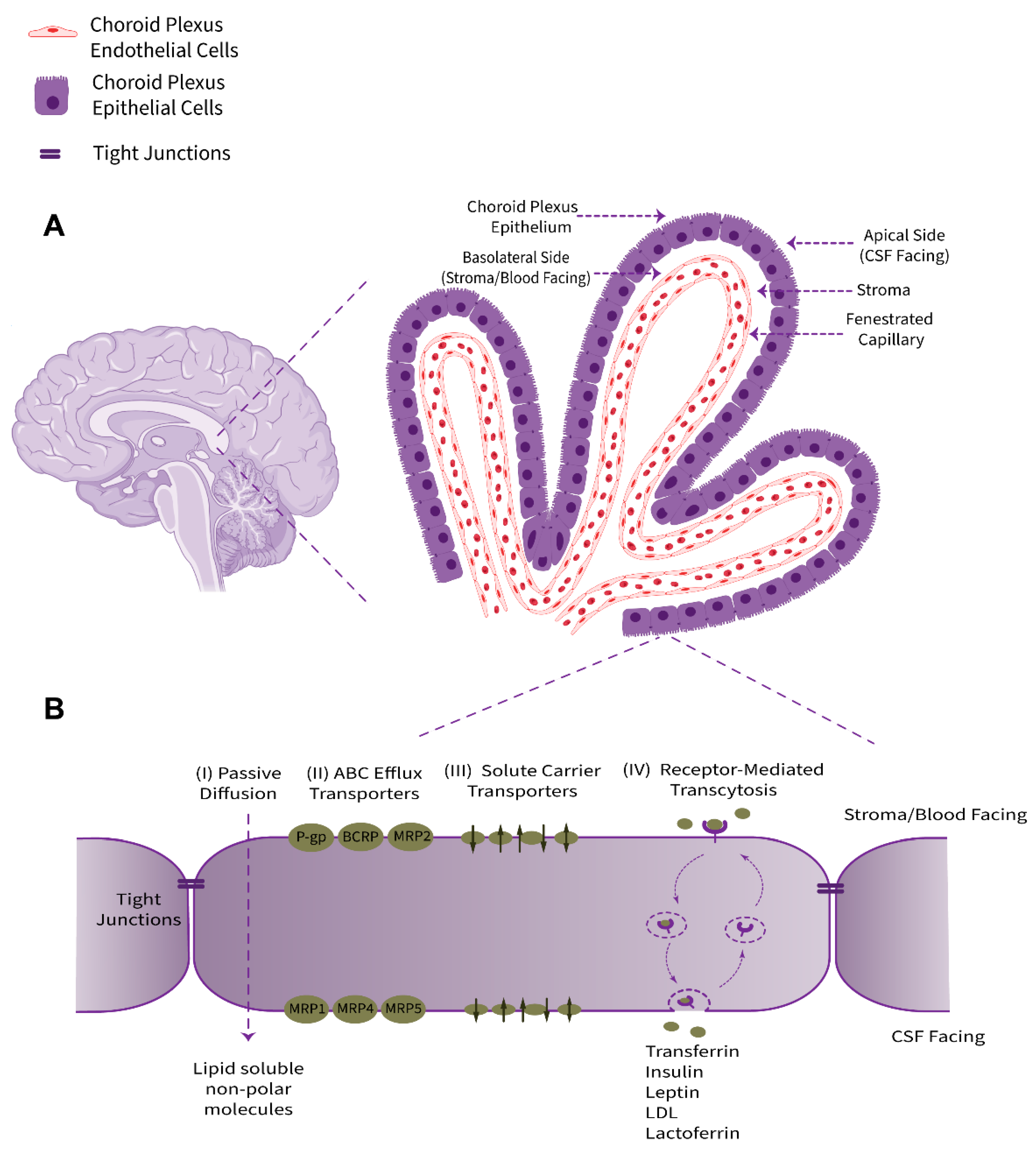
The choroid plexus, as a villous connective and highly vascularized structure, is the major component of the BCSFB system that connects the two principal physiological circulatory systems, namely the peripheral blood circulation and the CSF bulk flow. The choroid plexus convolute consists of two tissue layers, an outer secretory roughly cuboidal epithelium and an inner underlying stromal core comprising an immense network of fenestrated leaky blood vessels with a rich extracellular matrix. Cellular and sub-cellular foundations of choroid plexus barrier properties are non-neuronal epithelial cells (defined as a subtype of macroglia, derived from neuroectoderm) and their adjacent TJs (Figure 1). From an anatomical point of view, this ventricular structure is protruded in the brain lateral, third, and fourth ventricles. The choroid plexus epithelium is in line with the ependyma, a cuboidal epithelium interconnected by gap junctions covering the lumen of the cerebral ventricles; however, despite sharing a common embryological origin, these two cell types are quite distinct [4].

Figure 1. Schematic representation of anatomical location, physiological properties, and pharmacologically related transport systems of the BCSFB. (A) The BCSFB structure is comprised of the choroid plexus polarized cuboidal epithelial cells surrounding the highly permeable fenestrated capillaries of stromal core and tight junctional strands uniting adjacent epithelial cells. The innermost capillary (containing red blood cells), with leaky inter-endothelial gap junctions, is alongside the underlying stroma/basement membrane extracellular matrix. The epithelial basolateral surface faces stroma/blood and is in contact with interstitial fluid (ISF). The brush border apical membrane containing microvilli faces the adjacent CSF. (B) The main transport-relevant features of the BCSFB in terms of influx and efflux transport systems responsible for supplying nutrients, hormones, and therapeutics to the brain/CSF or acting to eliminate metabolites, xenobiotics, and neurotoxic compounds, respectively, are depicted.
The choroid plexus contributes to the production and secretion of CSF (approximately 500 mL per day in humans, or more precisely ~0.4 mL/min/g choroid plexus in adult mammals) and cerebral homeostasis by regulation of the blood–CSF exchange taking advantage of numerous transport systems allocated in a polarized configuration among the apical or brush border (luminal, CSF-facing) and the basolateral (abluminal, stroma-, or blood-facing) membranes of the choroidal epithelial cells [5]. The choroid plexus epithelium supplies micronutrients, hormones, growth factors, neurotrophins, and neuroprotective proteins to the CSF–brain nexus and uniquely provides micronutrients, such as ascorbic acid (vitamin C) and folate, for neuronal networks and glia [6][7][8][9]. Taken together, the choroid plexus acts in a complementary collaboration with brain microvessels to furnish regulatory factors and essential substances to meet the cerebral metabolism requirements.
3. Available Platforms
3.1. Static Monolayer Cultures Using Bicameral Systems
Basic 2D models are static systems of choroid plexus epithelial cell monocultures, predominantly based on semipermeable microporous membranes. The static culture systems are defined as those in which cultured cells are kept in the absence of physiological fluid dynamics. In this context of negligible fluid shear stress, the exchange of nutrients and waste takes place using diffusion [10]. These in vitro BCSFB epithelial barrier models have conventionally been founded on bicameral systems (alternatively called cell culture inserts), which possess a cell culture support fabricated from microporous permeable polymer membrane, generating distinct environments on opposing surfaces of a cell monolayer [11]. Upon cultivation, choroidal epithelial cells usually form a tight and polarized confluent monolayer, which can be regarded as a physiologically active cell culture model of the blood–CSF barrier. If cells are grown on top of the semipermeable membranes, this system’s upper or apical compartment simulates the ventricular space, and the basolateral/lower reservoir mimics the blood/stroma side. In the case of the inverted culture of epithelial cells on the lower face of inserts, such as in the method described by Dinner et al. [12], the apical and basolateral directions would be switched. In this manner, either transfer directions (blood-to-CSF and CSF-to-blood) are easily and independently accessible for further analyses.
The facile processing of filters makes this system an appropriate candidate for high-throughput screening (HTS) studies of drug permeability, binding affinity measurements, and transport kinetics. In general, this model type is well suited to explore in detail transport mechanisms (ranging from passive diffusion, facilitated transport, receptor-mediated transport) and cellular transmigration processes, and to investigate the permeability of potential drug candidates as well as the transepithelial resistance. Other applications include the analysis of the metabolism of molecules en passage and the determination of the ensuing metabolites [5].
3.2. Co-Culture Models
To generate a model resembling more closely the in vivo scenario, possessing functional TJs, high TEER, and expression of specific transporters and enzymes, co-culture systems can be developed [13][14][15][16][17]. To enhance the quality of BCSFB in vitro models, cell culture barrier models can be configured in complex co-culture systems, in which choroid plexus epithelial cells are grown on porous cell culture inserts alongside endothelial cells, mesenchymal (e.g., pericytes), and/or glial cells either cultivated on the bottom of a multi-well plate into which the insert is located (non-contact) or seeded on the opposite side of the inserts containing epithelial cells (leading to a so-called back-to-back contact co-culture).
3.3. 3D Cultures and Organoids
State-of-the-art three-dimensional (3D) culture systems, including matrix-based and matrix-free models, such as organoids and spheroids, have been recognized as the next advancement from static mono- and co-cultures. These 3D platforms with diverse features constitute promising alternatives to animal models and 2D cell culture systems in an in vitro tool to recapitulate the complex features of cerebral barriers [18][19]. In order to recapitulate the choroid plexus cytoarchitectural arrangement and a model resembling physiological conditions more closely, 3D cultures and organoids can be approached. Compared to other, less sophisticated in vitro counterparts, these models most accurately reflect the BCSFB properties and are valuable alternative tools to the use of animal subjects in CNS-oriented drug discovery programs.
3.3.1. 3D Explants and Cultured Cells in a Scaffold System
The simple design of appropriate cells cultured in a suitable scaffold or gel system, can be instrumental in the generation of high-throughput in vitro BCSFB models. In spite of few experimental experiences with the BCSFB, the currently available knowledge of BBB cells cultured in 3D platforms can be extended to the BCSFB field [20][21][22]. Three-dimensional explants of the choroid plexus can be generated from fragments of choroid plexuses, dissected from animals, human postmortem tissue, or human surgical samples cultured in suitable matrices, such as Matrigel™ and other commercially available hydrogels [23][24]. Three-dimensional explant platforms of mouse, rat, and human choroid plexus have been well prepared and maintained in the culture [23][25][26][27].
3.3.2. Organoids and Self-Organized 3D Models
Organoids, as defined by Huch et al., are 3D structures derived from either pluripotent stem cells or neonatal or adult stem/progenitor cells, in which cells spontaneously self-organize into properly differentiated functional cell types, and which recapitulates at least some function of the organ [28]. In the case of brain organoids, these accurate and versatile in vitro models reproduce several attributes of the brain barriers, including the expression of tight junctions, molecular transporters, and drug efflux pumps, and are critical tools for the study of brain barriers transport and the development of theranostics that can reach the CNS [29][30]. The potential to be scaled up to a high-throughput format, the ease of culture, and the miniature size nominate these multicellular organoids as robust, reliable, and predictive platforms to analyze and screen brain-penetrating compounds for the discovery of new and optimized treatment approaches for various neuropathologies.
Organoids of the choroid plexus as in vitro research platforms, derived from human pluripotent stem cells (human embryonic stem cells (ESCs) and induced pluripotent stem cells (iPSCs)) recapitulate fundamental morphological and functional attributes of this organ [31]. These sophisticated models overcome the disadvantage of species-to-species differences and can improve the understanding of the development/function of the human choroid plexus, which due to the lack of experimental access to this vital brain tissue, is still elusive.
3.3.3. Three-Dimensional Bioprinting Strategies
Three-dimensional bioprinting, as an additive manufacturing technology for modeling of user-defined biological samples, has emerged as a promising tool for the expansion of the BBB models. There is no known report of the in vitro 3D-bioprinted models of the BCSFB until now, but there are studied cases of the BBB [32][33][34][35][36], which have the potential to be adapted by the researchers in the field of the BCSFB. In principle, major printing modalities of inkjet-based, extrusion-based, and light-assisted bioprinting can be exploited to establish models with a high level of heterogeneity and biomimicry, which possess great potential as drug screening platforms [32].
3.4. Dynamic Models and Microfluidic Platforms
To generate experimental conditions largely comparable to the in vivo environment, the impact of hydrostatic pressure and fluid-induced shear stress can be incorporated into in vitro model platforms. In the light of this phenomenon and with further technological advances, in addition to static models, dynamic systems and culture in microfluidic chamber devices have been evolved, in which a tunable shear stress is induced by a continuous flow of culture medium, in either pump-based or pumpless dynamic configurations [10].
Microfluidic device-based models, or so-called organ-on-a-chip systems, are dynamic models with a precisely controlled periodic physiological fluid flow that tend to augment the survival of three-dimensional cultures and organoid models, with improved nutrients/wastes and oxygen exchange, and more realistic dimensions and geometries. Microfluidics-based approaches, as next generation drug testing tools, rely on spatially resolved compartments joined together via microgrooves, allowing cell-to-cell interactions, precise control of the 3D cellular and extracellular matrix, and the flow of small amounts of fluids [18]. The integration of functional organ elements onto these structures enables the study of multi-organ interactions and dynamics of drug activities [37]. The rationale is that these emerging in vitro model platforms reconstitute the choroid plexus-mimetic microenvironment more accurately, which enables effective modeling of this tissue for (neuro)therapeutics development and research. Furthermore, the advancement in the 3D printing technology, nanofabrication, integrated sensors, and the versatility of human-derived stem cells have contributed largely to boost the field [38][39]. However, the system setup is sophisticated, time-consuming, and requires specialized equipment, significant resources, and technical skills compared to conventional static models, leading to low-throughput screening capabilities and hindering their broad application. Despite the widespread use of dynamic models, such as cone–plate apparatuses, microporous hollow fibers, and microfluidic-based devices in the generation of in vitro BBB models [40][41], their application and usefulness remain to be adapted for their BCSFB counterparts.
4. Available Cells
4.1. Cerebral Originating Cells
The application of human-origin primary cells as in vitro models is restricted due to ethical and technical issues. Therefore, small and large animals have been sources of primary choroid plexus epithelium for various studies. Primary cultures of choroid plexus epithelial cells have been established from various species, including mouse [4][42][43][44][45], rat [46][47][48][49][50], pig [51][52][53][54][55][56][57][58][59][60][61], cow [62][63], sheep [64][65], rabbit [66][67][68], and non-human primates such as rhesus macaque [69]. Canine choroid plexus cells have been isolated as well, though being more challenging compared to other species [46].
Human primary choroid plexus epithelial cells can be obtained from aborted embryos, directly after surgical removal, or postmortem [3][70][71]. However, postmortem-derived human samples may have disturbed viability and functionality depending on the time elapsed after death and could be impacted by the health status of the subject in terms of infections, disorders, injuries, and medication history [3][72][73], and, due to limited applicability for functional assays and usual vectorial transport studies, are gradually abandoned by the researchers in the course of time. HCPEpiC from ScienCell Research Laboratories (also obtainable from other companies) are another human source for primary epithelial cells of the choroid plexus [74][75]. According to the manufacturer, further expansion of these cell populations for 15 doublings is guaranteed. However, multiple concerns are still inherited by this commercially available culture in terms of its origin, nature, and morphology [3].
The experimental procedure to isolate primary choroid plexus epithelial cells from different species and establish a pure cell culture devoid of contaminating cell types has been described comprehensively elsewhere and is not brought up here for the sake of brevity [4][76][77]. Principally, the freshly isolated primary cells retain the differentiated properties of the choroidal epithelium and exhibit a myriad of morphological- and biochemical-desired properties, yet the disadvantages are a small culture size, a limited proliferative potential, and a diminished viability over time after dissection. Therefore, prompt transfer to a suitable in vitro setting is crucial for establishing viable cultures [4].
Z310 and TR-CSFB cells, as rat immortalized cell lines carrying the simian virus 40 large T-antigen gene, are well-characterized choroid plexus epithelial cells that have found their way as suitable cells [78][79][80][81][82][83][84][85]. TR-CSFB cells (five cell lines, TR-CSFB 1–5) exhibit a polygonal-shaped morphology comparable to primary cultured rat choroid plexus epithelial cells. Immunohistochemically, the TR-CSFB cell lines express TTR, apically located Na+, K+-ATPase, and the efflux transporters ABCB1/MDR1a, ABCC1/MRP1, and ABCG2/BCRP [86].
Murine choroid plexus carcinoma cell lines ECPC3 and ECPC4 are isolated from transgenic mice harboring the viral simian virus 40 large T oncogene under the transcriptional control of an intronic enhancer region from the human immunoglobulin heavy chain gene. These cells have shown acceptable stability in culture in a time span of a year [87][88].
The PCP-R cell line has been established based on primary porcine choroid plexus epithelial cells (PCPEC). The cell line exhibits a regular polygonal pattern, expresses junctional proteins, and develops morphologically dense TJs. When cultured on cell culture inserts, this cell line exhibits characteristic barrier properties of TEER above 600 Ω × cm2 and a restricted permeability for macromolecular paracellular markers [89][90].
SCP, a sheep choroid plexus epithelial cell line, is another alternative option on hand [8][91][92][93][94][95][96]. A variant of this ovine finite cell line (prepared from brain choroid plexus of Ovis aries) is listed in the American Type Culture Collection (ATCC) as cell line SCP No. CRL-1700®, or as catalog number 89101302 in the European Collection of Authenticated Cell Cultures (ECACC).
4.2. Noncerebral-Based Cells (Surrogate Models)
Noncerebral-originating cells are considered surrogates in blood–CSF barrier modeling. Madin-Darby canine kidney (MDCK and MDCKII) cells, human colonic epithelial cell line Caco-2 cells, Ralph Russ canine kidney cells (RRCK), Lilly Laboratories Culture-Porcine Kidney 1 epithelial cells (LLC-PK1), and cells transfected with specific efflux transporters or pharmacologic targets can be employed as a surrogate cell-based model to assess the permeability of selected compounds in the presence and absence of overexpressed efflux transporters or to evaluate their mechanism of action and effectiveness [97][98][99][100][101][102][103][104]. The spontaneously immortalized MDCK-MDR1 cell line expressing P-gp/ABCB1, MDCK2-ABCB1 cells expressing ABCB1, and LLC-PK1/BCRP cells expressing the efflux transporter ABCG2/BCRP are examples of surrogate cells, which can be used [105].
5. Models Validation Criteria
5.1. Barrier Morphology
Once confluent, an in vitro choroid plexus epithelial cell barrier represents a differentiated polarized morphology, whose ultrastructure can be assessed using standard imaging modalities, including immunofluorescence microscopy and TEM. The latter allows direct visualization of the cells, their organizational structure, and any possible defect or imperfection in the cellular monolayer. Nonetheless, only few studies have used morphology imaging of in vitro cellular barriers as a routine quality validation to assure a closeness to in vivo conditions [106].
5.2. Barrier Properties
The reproducible tightness of models can be ascertained by convenient measuring of the TEER. The TEER value is a well-acknowledged measure to appraise the ion permeability of cell layers, reflecting the passive conductance of the TJs to small inorganic electrolytes, and impedance analysis manifests the electrical capacitance of the barrier likewise. Since the TEER can be correlated to the amount and degree of complexity of functional TJs and the expression of microvilli and other membrane invaginations, a high TEER might reflect the resemblance to in vivo situations. As a non-invasive quantitative method, the TEER measurement provides the most selective approach towards evaluating barrier integrity [59][107][108].
5.3. Exogenous Tracer Permeability
Exogenous tracers or inert paracellular flux markers compatible with analytical conditions can provide beneficial information on the permeability status of model barriers towards lipid insoluble organic compounds. However, one should be aware of potential side effects and avoid unnecessary interactions of the tracers with experimental elements. Exogenous tracers come in various physicochemical properties, ranging from proteins and polysaccharides to small polar compounds (such as, but not limited to, mannitol, sucrose, inulin, and dextran) [109]. Most routinely used protein markers are purified plasma proteins such as bovine albumin (~66 kDa), human albumin (~66 kDa), bovine fetuin (~49 kDa), and horseradish peroxidase (40 kDa). It should always be noted that the choice of the protein marker relies upon the biological process being studied and the experimental design. Still, it should be antigenically distinguished from the cells under study. A wide selection of dextran conjugates in a diverse molecular weight range (MW 3, 10, 40, 70, 150, 500, and 2000 kDa) is also available. Owing to satisfactory water solubility, low toxicity, limited immunogenicity, and biologically inertness, dextrans are among the most popular exogenous tracers used to determine barrier tightness.
5.4. Functional Junctional Proteins and Transporters
Analysis of the expression and localization of junctional proteins and relevant carriers/transporters and their accordance with the choroidal epithelium in vivo can reveal information regarding the quality index of the model. Depending on the individual experimental goal, the characterization of specific markers, enzymes, receptors, adhesion molecules, and specific proteins/polypeptides, can be performed. Immunostaining of cell-type-specific markers and junctional molecules leads to a qualitative confirmation of barrier integrity of an epithelial monolayer.
To assess the efflux transporters’ activity, substrate accumulation assays are exploited. Various efflux inhibitors (such as cyclosporin A and MK-571) are used to perform such substrate accumulation assays, assuming that substrate-inhibitor pairs are appropriately matched for each efflux transporter. In this assay configuration, model cells are incubated with the desired substrate either with or without their respective inhibitors, and at the culmination of the experiment, normalized fluorescence of the cells is calculated. In the case of the functional expression of transporters, the uptake of substrate should be increased in the presence of a corresponding transporter inhibitor [110].
Directional transport: in another experiment variation, transporter activities can be evaluated using directional transport assays, in which inhibitors are only added on the side of the cells grown on cell culture inserts where directional transport is being assessed. Here, again, model cells are incubated with the desired substrate either with or without their respective inhibitors, and the fluorescence signal on the opposite filter side is measured. If efflux transporters are expressed functionally at apical or basolateral surfaces, then the directional transport of substrate in the presence of the corresponding inhibitor is enhanced [110].
5.5. Factors Critical to Cell Selection and Culture Conditions
Serum withdrawal also seems to impact choroid plexus cells to reach the full barrier function. Serum deprivation can tighten the epithelial monolayer and improve the cellular polarity [76][111]. However, the barrier-dismantling effect of serum is believed to be compartment-specific, meaning that it depends on the apical or basolateral exposure of the cell layer to serum. In the case of serum exposure to the apical (comparable to CSF side in vivo) surface of the choroid plexus epithelial cell monolayer, the barrier function is diminished vastly. On the other hand, when serum is applied to the basolateral (corresponding to the stroma/blood side in vivo) surface, the epithelial barrier function is scarcely affected [51][61].
Due to the fact that barrier properties of epithelia are modulated by cAMP-dependent pathways, the presence of membrane-permeable cAMP analogs such as 8-(4-chlorophenylthio)-cAMP (CPT-cAMP) or the adenylate cyclase activator forskolin can have an augmenting effect on TEER values of choroid plexus epithelial cell layers [51][112][113].
Corticosteroids (hydrocortisone, dexamethasone) application to in vitro models may increase the barrier tightness of the epithelial and endothelial cells monolayer. This phenomenon is proposed to be a consequence of the regulation of the expression and distribution of tight junction proteins upon corticosteroids treatment [114]. Dexamethasone, as a synthetic glucocorticoid, has been shown to improve barrier strength and can be exploited as a positive control to investigate the effect of various conditions on the barrier integrity [115][116][117].
6. Applications in (Neuro)Therapeutics Development and Research
6.1. Permeability Screenings and Studies
Any rational drug discovery project dealing with candidates targeted to the brain or requiring exclusion from the CNS to prevent possible side effects should contemplate permeability measurements and prediction studies. Hence, profiling brain/CSF permeability of investigational novel molecular species at preliminary stages of the drug development track is game-changing. To this end, models can predict the CNS permeability of novel or known compounds in relation to both the route and rate.
Models expressing the specific transporters found in choroid plexus epithelium could be harnessed to establish whether the permeation of a compound of interest is impacted by a specific carrier system (e.g., P-gp) and to provide comprehensive knowledge on the physiology and modulation of such transporters. From another point of view, several neurological disorders are assumed to be associated with the dysfunction of transporters in the brain, and the detailed region-specific knowledge of transporters and their interaction with investigational therapeutics can prove helpful. In the case that transporters govern the permeability or uptake of a test compound, Michaelis–Menten kinetics can be obtained using nonlinear regression analysis of the concentration dependence influx. In vitro models have proven advantageous and constructive in studies of thyroxine [119], leptin [120], taurine [68], ascorbic acid [56], creatinine [121], and the neuroactive flavonoid resveratrol [122] transport across epithelial cells of choroid plexus. The in vitro BCSFB models have also proven helpful in library screening and identification of cell specific penetrating peptides for the choroid plexus epithelium using phage display techniques [27][123].6.2. Transport Mechanisms Studies and (Targeted)Drug Delivery
Thanks to their physicochemical nature, hydrophobic/lipophilic substances of a low molecular weight are capable of free diffusion across cell membranes. In contrast, as mentioned in previous sections, the unrestricted diffusion of hydrophilic chemical species is substantially hindered due to the effective closure and sealing of the paracellular shunt by TJs. Accordingly, access to the CSF is granted exclusively to those compounds that are transported actively by the corresponding transport systems in the plasma membrane of choroid plexus epithelial cells. Theoretically, therapeutic- and diagnostic agents that are effectively and successfully transported by the choroid plexus and remain to some extent unaffected by the metabolizing enzymes and efflux transporters are rapidly distributed throughout the CNS using the bulk flow of CSF. This is due to the fact that at the brain ventricles, the extracellular/interstitial fluid (ISF) and the CSF are separated from each other by the non-barrier/non-restrictive permeable layer of ependymal cells, leading to a direct continuity of these two components and the subsequent free exchange of substances within the extracellular space of CNS [118].
Therapeutic and diagnostic agents can pass the BCSFB via the transcellular route by employing one of the possible pathways, which are passive diffusion, facilitated diffusion, or vesicular transfer or transcytosis mechanisms. Generally, cellular uptake mechanisms based on endocytosis/transcytosis are the preferred cell entry route for many compounds. The endocytosis process can be categorized into two broad divisions of phagocytosis and pinocytosis. While the former is restricted to specialized cell types, the latter occurs in all cell types and can be subdivided into macropinocytosis, clathrin-dependent endocytosis (CDE), and clathrin-independent endocytosis (CIE) [124]. Contrary to endocytosis, transcytosis mechanisms are not well understood. Endocytosis and transcytosis across choroid plexus epithelium, studied at the subcellular level, can shed light on mechanisms of targeted delivery of therapeutics across this target site. These studies may rely on endocytosis inhibitors (including, but not limited to, chlorpromazine, genistein, methyl-β-cyclodextrin, and potassium depletion) to provide information concerning the endocytic pathway of compounds under study.
6.3. Metabolites/Xenobiotics Transport(er) Regulation
The fate of drugs and xenobiotics metabolites, affiliated to several chemical classes, generated at choroidal epithelial cells and the relative contribution of various efflux pumps present at this barrier can also be investigated using these in vitro models. In addition, the potential effect of multiple metabolites, xenobiotics, and compounds on both influx into and efflux out of the CSF can be studied. Referring to static bicameral device models, efflux transport assays can be performed, and the efflux ratio is estimated by dividing the permeability value in the apical to basolateral (A−B) direction by the permeability value in the basolateral to apical (B−A) direction. With respect to efflux transport assays, the transport of the compounds under study is assessed in the presence of inhibitors of relevant transporter proteins and compared to the non-inhibited conditions. Namely, verapamil, GF120918, PSC833, and N-desmethyl-loperamide as inhibitors of ABCB1/P-gp, Ko143, and fumitremorgin C as an inhibitor of ABCG2/BCRPs, tariquidar and elacridar as inhibitors of both P-gp and BCRP, or MK571 as an inhibitor of various MRPs are examples that can be applied for such experiments [125]. As an alternative strategy, the inhibition of these efflux transporters potentially improves the pharmacokinetics of CNS therapeutic candidates. To this end, the efficacy of a range of compounds (mainly phytoestrogens) as modulators of BCRP/ABCG2 has been evaluated using in vitro BCSFB models [126]. Accordingly, genetic manipulation approaches, such as overexpression, knock-out, or knock-down of specific transporters can be approached.
6.4. In Vitro Molecular Verification of Pharmacological Activity
One of the main thrusts behind the development of in vitro BCSFB models has been to shed light on the pharmacological molecular mechanisms of compounds toward this interface. Using cell-based BCSFB models, in vitro activity, potency, and mechanisms of actions of investigational CNS therapeutics and compounds under study can be demonstrated in preclinical drug evaluations. Excerpts of case studies from distinct pharmacological categories describe investigations of the anti-inflammatory activity of synthetic matrix metalloproteinase (MMP) inhibitors [43], and events of receptor activation for drugs of potential abuse and hallucinogens have been studied [127].
6.5. (Neuro)Toxicological Studies
Harnessing appropriate in vitro BCSFB models, the ever-increasing concern regarding hazardous compounds, such as environmental toxins, metals, pesticides, solvents, as well as the broad field of study of neurotoxicants, can be the target of both exposure (amounts permeated) and impact (effects exerted) investigations. The models have proven valuable in evaluating the influx/efflux of (neuro)toxins, understanding the associated neuroprotective mechanisms, elucidating the alteration of the BCSFB function by toxic compounds, and the involvement of the BCSFB in neuroinflammation [128].
6.6. Pharmacological Interventions at the BCSFB
Transport data acquired through pharmacological interventive measures and modulation by hormones and drugs contribute to understanding the BCSFB homeostatic phenomena and barrier/transporting functions. In addition, establishing approaches for restoring aberrant choroid plexus and CSF dynamics during disease has been of great interest to researchers in pharmacological, neurological, and neurosurgical fields.
6.7. The BCSFB and Choroid Plexus as a Drug Target in Various Diseases
Choroid plexus breakdown has been proposed and hypothesized in a wide range of neurological conditions, including aging, Alzheimer’s disease, Parkinson’s disease, epilepsy, stroke, neoplasms, perhaps psychiatric disorders, intracranial hypertension, and certainly varying types of hydrocephalus. Therefore, targeting this tissue may offer opportunities to translate neurotherapeutics from the lab to the clinic, and provide insights toward rational drug therapy to restore barrier function [129][130][131][132].
This entry is adapted from the peer-reviewed paper 10.3390/pharmaceutics14081729
References
- Spector, R.; Keep, R.F.; Snodgrass, S.R.; Smith, Q.R.; Johanson, C.E. A balanced view of choroid plexus structure and function: Focus on adult humans. Exp. Neurol. 2015, 267, 78–86.
- Johanson, C.E.; Duncan, J.A.; Stopa, E.G.; Baird, A. Enhanced prospects for drug delivery and brain targeting by the choroid plexus—CSF route. Pharm. Res. 2005, 22, 1011–1037.
- Redzic, Z.B. Studies on the human choroid plexus in vitro. Fluids Barriers CNS 2013, 10, 10.
- Menheniott, T.R.; Charalambous, M.; Ward, A. Derivation of primary choroid plexus epithelial cells from the mouse. Methods Mol. Biol. 2010, 633, 207–220.
- Strazielle, N.; Preston, J.E.; Nag, S. Transport across the choroid plexuses in vivo and in vitro. Methods Mol. Med. 2003, 89, 291–304.
- Johanson, C.E.; Keep, R.F. Blending established and new perspectives on choroid plexus-CSF dynamics. In Role of the Choroid Plexus in Health and Disease; Praetorius, J., Blazer-Yost, B., Damkier, H., Eds.; Springer: New York, NY, USA, 2020; pp. 35–81.
- Spector, R.; Johanson, C.E. The nexus of vitamin homeostasis and DNA synthesis and modification in mammalian brain. Mol. Brain 2014, 7, 3.
- Gee, P.; Rhodes, C.H.; Fricker, L.D.; Angeletti, R.H. Expression of neuropeptide processing enzymes and neurosecretory proteins in ependyma and choroid plexus epithelium. Brain Res. 1993, 617, 238–248.
- Stopa, E.G.; Berzin, T.M.; Kim, S.; Song, P.; Kuo-LeBlanc, V.; Rodriguez-Wolf, M.; Baird, A.; Johanson, C.E. Human choroid plexus growth factors: What are the implications for CSF dynamics in Alzheimer’s disease? Exp. Neurol. 2001, 167, 40–47.
- Tan, H.Y.; Cho, H.; Lee, L.P. Human mini-brain models. Nat. Biomed. Eng. 2021, 5, 11–25.
- Larsen, J.B.; Taebnia, N.; Dolatshahi-Pirouz, A.; Eriksen, A.Z.; Hjørringgaard, C.; Kristensen, K.; Larsen, N.W.; Larsen, N.B.; Marie, R.; Mündler, A.K.; et al. Imaging therapeutic peptide transport across intestinal barriers. RSC Chem. Biol. 2021, 2, 1115–1143.
- Dinner, S.; Borkowski, J.; Stump-Guthier, C.; Ishikawa, H.; Tenenbaum, T.; Schroten, H.; Schwerk, C. A choroid plexus epithelial cell-based model of the human blood-cerebrospinal fluid barrier to study bacterial infection from the basolateral side. J. Vis. Exp. 2016, 11, 54061.
- Dehouck, M.P.; Méresse, S.; Delorme, P.; Fruchart, J.C.; Cecchelli, R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J. Neurochem. 1990, 54, 1798–1801.
- Gaillard, P.J.; Voorwinden, L.H.; Nielsen, J.L.; Ivanov, A.; Atsumi, R.; Engman, H.; Ringbom, C.; de Boer, A.G.; Breimer, D.D. Establishment and functional characterization of an in vitro model of the blood-brain barrier, comprising a co-culture of brain capillary endothelial cells and astrocytes. Eur. J. Pharm. Sci. 2001, 12, 215–222.
- Reichel, A.; Begley, D.J.; Abbott, N.J. An overview of in vitro techniques for blood-brain barrier studies. Methods Mol. Med. 2003, 89, 307–324.
- Di Marco, A.; Gonzalez Paz, O.; Fini, I.; Vignone, D.; Cellucci, A.; Battista, M.R.; Auciello, G.; Orsatti, L.; Zini, M.; Monteagudo, E.; et al. Application of an in vitro blood-brain barrier model in the selection of experimental drug candidates for the treatment of Huntington’s disease. Mol. Pharm. 2019, 16, 2069–2082.
- Deli, M.A.; Abrahám, C.S.; Kataoka, Y.; Niwa, M. Permeability studies on in vitro blood-brain barrier models: Physiology, pathology, and pharmacology. Cell Mol. Neurobiol. 2005, 25, 59–127.
- von Maydell, D.; Jorfi, M. A synergistic engineering approach to build human brain spheroids. Methods Mol. Biol. 2021, 2258, 151–169.
- Logan, S.; Arzua, T.; Canfield, S.G.; Seminary, E.R.; Sison, S.L.; Ebert, A.D.; Bai, X. Studying human neurological disorders using induced pluripotent stem cells: From 2D monolayer to 3D organoid and blood brain barrier models. Compr. Physiol. 2019, 9, 565–611.
- Ahn, S.I.; Sei, Y.J.; Park, H.J.; Kim, J.; Ryu, Y.; Choi, J.J.; Sung, H.J.; MacDonald, T.J.; Levey, A.I.; Kim, Y. Microengineered human blood-brain barrier platform for understanding nanoparticle transport mechanisms. Nat. Commun. 2020, 11, 175.
- Katt, M.E.; Linville, R.M.; Mayo, L.N.; Xu, Z.S.; Searson, P.C. Functional brain-specific microvessels from iPSC-derived human brain microvascular endothelial cells: The role of matrix composition on monolayer formation. Fluids Barriers CNS 2018, 15, 7.
- Potjewyd, G.; Kellett, K.A.B.; Hooper, N.M. 3D hydrogel models of the neurovascular unit to investigate blood-brain barrier dysfunction. Neuronal Signal. 2021, 5, Ns20210027.
- Petersen, N.; Torz, L.; Jensen, K.H.R.; Hjortø, G.M.; Spiess, K.; Rosenkilde, M.M. Three-dimensional explant platform for studies on choroid plexus epithelium. Front. Cell. Neurosci. 2020, 14, 108.
- Ulloa, V.; Saldivia, N.; Ferrada, L.; Salazar, K.; Martínez, F.; Silva-Alvarez, C.; Magdalena, R.; Oviedo, M.J.; Montecinos, H.; Torres-Vergara, P.; et al. Basal sodium-dependent vitamin C transporter 2 polarization in choroid plexus explant cells in normal or scorbutic conditions. Sci. Rep. 2019, 9, 14422.
- Pellegrini, L.; Lancaster, M.A. Breaking the barrier: In vitro models to study choroid plexus development. Curr. Opin. Cell. Biol. 2021, 73, 41–49.
- Dragunow, M.; Feng, S.; Rustenhoven, J.; Curtis, M.; Faull, R. Studying human brain inflammation in leptomeningeal and choroid plexus explant cultures. Neurochem. Res. 2016, 41, 579–588.
- Gonzalez, A.M.; Leadbeater, W.E.; Burg, M.; Sims, K.; Terasaki, T.; Johanson, C.E.; Stopa, E.G.; Eliceiri, B.P.; Baird, A. Targeting choroid plexus epithelia and ventricular ependyma for drug delivery to the central nervous system. BMC Neurosci. 2011, 12, 4.
- Huch, M.; Knoblich, J.A.; Lutolf, M.P.; Martinez-Arias, A. The hope and the hype of organoid research. Development 2017, 144, 938–941.
- Bergmann, S.; Lawler, S.E.; Qu, Y.; Fadzen, C.M.; Wolfe, J.M.; Regan, M.S.; Pentelute, B.L.; Agar, N.Y.R.; Cho, C.F. Blood-brain-barrier organoids for investigating the permeability of CNS therapeutics. Nat. Protoc. 2018, 13, 2827–2843.
- Simonneau, C.; Duschmalé, M.; Gavrilov, A.; Brandenberg, N.; Hoehnel, S.; Ceroni, C.; Lassalle, E.; Kassianidou, E.; Knoetgen, H.; Niewoehner, J.; et al. Investigating receptor-mediated antibody transcytosis using blood-brain barrier organoid arrays. Fluids Barriers CNS 2021, 18, 43.
- Jacob, F.; Pather, S.R.; Huang, W.K.; Zhang, F.; Wong, S.Z.H.; Zhou, H.; Cubitt, B.; Fan, W.; Chen, C.Z.; Xu, M.; et al. Human pluripotent stem cell-derived neural cells and brain organoids reveal SARS-CoV-2 neurotropism predominates in choroid plexus epithelium. Cell Stem Cell 2020, 27, 937–950.e939.
- Tang, M.; Rich, J.N.; Chen, S. Biomaterials and 3D bioprinting strategies to model glioblastoma and the blood-brain barrier. Adv. Mater. 2021, 33, e2004776.
- Marino, A.; Tricinci, O.; Battaglini, M.; Filippeschi, C.; Mattoli, V.; Sinibaldi, E.; Ciofani, G. A 3D real-scale, biomimetic, and biohybrid model of the blood-brain barrier fabricated through two-photon lithography. Small 2018, 14, 1702959.
- Kim, J.A.; Kim, H.N.; Im, S.K.; Chung, S.; Kang, J.Y.; Choi, N. Collagen-based brain microvasculature model in vitro using three-dimensional printed template. Biomicrofluidics 2015, 9, 024115.
- Qi, D.; Wu, S.; Lin, H.; Kuss, M.A.; Lei, Y.; Krasnoslobodtsev, A.; Ahmed, S.; Zhang, C.; Kim, H.J.; Jiang, P.; et al. Establishment of a human iPSC- and nanofiber-based microphysiological blood-brain barrier system. ACS Appl. Mater. Interfaces 2018, 10, 21825–21835.
- Galpayage Dona, K.N.U.; Hale, J.F.; Salako, T.; Anandanatarajan, A.; Tran, K.A.; DeOre, B.J.; Galie, P.A.; Ramirez, S.H.; Andrews, A.M. The use of tissue engineering to fabricate perfusable 3D brain microvessels in vitro. Front. Physiol. 2021, 12, 715431.
- Wang, Y.I.; Abaci, H.E.; Shuler, M.L. Microfluidic blood-brain barrier model provides in vivo-like barrier properties for drug permeability screening. Biotechnol. Bioeng. 2017, 114, 184–194.
- Musafargani, S.; Mishra, S.; Gulyás, M.; Mahalakshmi, P.; Archunan, G.; Padmanabhan, P.; Gulyás, B. Blood brain barrier: A tissue engineered microfluidic chip. J. Neurosci. Methods 2020, 331, 108525.
- Oddo, A.; Peng, B.; Tong, Z.; Wei, Y.; Tong, W.Y.; Thissen, H.; Voelcker, N.H. Advances in microfluidic blood-brain barrier (BBB) models. Trends Biotechnol. 2019, 37, 1295–1314.
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-vitro blood-brain barrier models for drug screening and permeation studies: An overview. Drug Des. Dev. Ther. 2019, 13, 3591–3605.
- Yu, F.; Selva Kumar, N.D.; Choudhury, D.; Foo, L.C.; Ng, S.H. Microfluidic platforms for modeling biological barriers in the circulatory system. Drug Discov. Today 2018, 23, 815–829.
- Thomas, T.; Stadler, E.; Dziadek, M. Effects of the extracellular matrix on fetal choroid plexus epithelial cells: Changes in morphology and multicellular organization do not affect gene expression. Exp. Cell Res. 1992, 203, 198–213.
- Aerts, J.; Vandenbroucke, R.E.; Dera, R.; Balusu, S.; Van Wonterghem, E.; Moons, L.; Libert, C.; Dehaen, W.; Arckens, L. Synthesis and validation of a hydroxypyrone-based, potent, and specific matrix metalloproteinase-12 inhibitor with anti-inflammatory activity in vitro and in vivo. Mediat. Inflamm. 2015, 2015, 510679.
- Barkho, B.Z.; Monuki, E.S. Proliferation of cultured mouse choroid plexus epithelial cells. PLoS ONE 2015, 10, e0121738.
- Péraldi-Roux, S.; Nguyen-Than Dao, B.; Hirn, M.; Gabrion, J. Choroidal ependymocytes in culture: Expression of markers of polarity and function. Int. J. Dev. Neurosci. 1990, 8, 575–588.
- Zheng, W.; Zhao, Q. The blood-CSF barrier in culture: Development of a primary culture and transepithelial transport model from choroidal epithelial cells. Methods Mol. Biol. 2002, 188, 99–114.
- Zheng, W.; Zhao, Q.; Graziano, J.H. Primary culture of choroidal epithelial cells: Characterization of an in vitro model of blood-CSF barrier. Vitr. Cell Dev. Biol. Anim. 1998, 34, 40–45.
- Watson, J.A.; Elliott, A.C.; Brown, P.D. Serotonin elevates intracellular Ca2+ in rat choroid plexus epithelial cells by acting on 5-HT2C receptors. Cell Calcium 1995, 17, 120–128.
- Batisson, M.; Strazielle, N.; Hejmadi, M.; Thomas, D.; Ghersi-Egea, J.F.; Etienne, J.; Vandenesch, F.; Lina, G. Toxic shock syndrome toxin-1 challenges the neuroprotective functions of the choroidal epithelium and induces neurotoxicity. J. Infect. Dis. 2006, 194, 341–349.
- Villalobos, A.R.; Parmelee, J.T.; Pritchard, J.B. Functional characterization of choroid plexus epithelial cells in primary culture. J. Pharmacol. Exp. Ther. 1997, 282, 1109–1116.
- Angelow, S.; Wegener, J.; Galla, H.-J. 4—Transport and permeability characteristics of the blood-cerebrospinal fluid barrier in vitro. In Blood-Spinal Cord and Brain Barriers in Health and Disease; Sharma, H.S., Westman, J., Eds.; Academic Press: San Diego, CA, USA, 2004; pp. 33–45.
- Haselbach, M.; Wegener, J.; Decker, S.; Engelbertz, C.; Galla, H.J. Porcine choroid plexus epithelial cells in culture: Regulation of barrier properties and transport processes. Microsc. Res. Tech. 2001, 52, 137–152.
- Hakvoort, A.; Haselbach, M.; Galla, H.J. Active transport properties of porcine choroid plexus cells in culture. Brain Res. 1998, 795, 247–256.
- Nilsson, C.; Fahrenkrug, J.; Lindvall-Axelsson, M.; Owman, C. Epithelial cells purified from choroid plexus have receptors for vasoactive intestinal polypeptide. Brain Res. 1991, 542, 241–247.
- Angelow, S.; Zeni, P.; Galla, H.J. Usefulness and limitation of primary cultured porcine choroid plexus epithelial cells as an in vitro model to study drug transport at the blood—CSF barrier. Adv. Drug Deliv. Rev. 2004, 56, 1859–1873.
- Angelow, S.; Haselbach, M.; Galla, H.J. Functional characterisation of the active ascorbic acid transport into cerebrospinal fluid using primary cultured choroid plexus cells. Brain Res. 2003, 988, 105–113.
- Angelow, S.; Zeni, P.; Höhn, B.; Galla, H.J. Phorbol ester induced short- and long-term permeabilization of the blood—CSF barrier in vitro. Brain Res. 2005, 1063, 168–179.
- Tenenbaum, T.; Essmann, F.; Adam, R.; Seibt, A.; Jänicke, R.U.; Novotny, G.E.; Galla, H.J.; Schroten, H. Cell death, caspase activation, and HMGB1 release of porcine choroid plexus epithelial cells during Streptococcus suis infection in vitro. Brain Res. 2006, 1100, 1–12.
- Baehr, C.; Reichel, V.; Fricker, G. Choroid plexus epithelial monolayers—A cell culture model from porcine brain. Cerebrospinal Fluid Res. 2006, 3, 13.
- Gath, U.; Hakvoort, A.; Wegener, J.; Decker, S.; Galla, H.J. Porcine choroid plexus cells in culture: Expression of polarized phenotype, maintenance of barrier properties and apical secretion of CSF-components. Eur. J. Cell Biol. 1997, 74, 68–78.
- Hakvoort, A.; Haselbach, M.; Wegener, J.; Hoheisel, D.; Galla, H.J. The polarity of choroid plexus epithelial cells in vitro is improved in serum-free medium. J. Neurochem. 1998, 71, 1141–1150.
- Crook, R.B.; Kasagami, H.; Prusiner, S.B. Culture and characterization of epithelial cells from bovine choroid plexus. J. Neurochem. 1981, 37, 845–854.
- Crook, R.B.; Prusiner, S.B. Vasoactive intestinal peptide stimulates cyclic AMP metabolism in choroid plexus epithelial cells. Brain Res. 1986, 384, 138–144.
- Holm, N.R.; Hansen, L.B.; Nilsson, C.; Gammeltoft, S. Gene expression and secretion of insulin-like growth factor-II and insulin-like growth factor binding protein-2 from cultured sheep choroid plexus epithelial cells. Brain Res. Mol. Brain Res. 1994, 21, 67–74.
- Salvatori, D.; Vincenzetti, S.; Maury, G.; Gosselin, G.; Gaubert, G.; Vita, A. Maedi-visna virus, a model for in vitro testing of potential anti-HIV drugs. Comp. Immunol. Microbiol. Infect. Dis. 2001, 24, 113–122.
- Ishiwata, I.; Ishiwata, C.; Ishiwata, E.; Sato, Y.; Kiguchi, K.; Tachibana, T.; Hashimoto, H.; Ishikawa, H. Establishment and characterization of a human malignant choroids plexus papilloma cell line (HIBCPP). Hum. Cell 2005, 18, 67–72.
- Ramanathan, V.K.; Hui, A.C.; Brett, C.M.; Giacomini, K.M. Primary cell culture of the rabbit choroid plexus: An experimental system to investigate membrane transport. Pharm. Res. 1996, 13, 952–956.
- Ramanathan, V.K.; Chung, S.J.; Giacomini, K.M.; Brett, C.M. Taurine transport in cultured choroid plexus. Pharm. Res. 1997, 14, 406–409.
- Delery, E.C.; MacLean, A.G. Culture model for non-human primate choroid plexus. Front. Cell. Neurosci. 2019, 13, 396.
- Greenwood, S.; Swetloff, A.; Wade, A.M.; Terasaki, T.; Ferretti, P. Fgf2 is expressed in human and murine embryonic choroid plexus and affects choroid plexus epithelial cell behaviour. Cerebrospinal Fluid Res. 2008, 5, 20.
- Swetloff, A.; Ferretti, P. Changes in E2F5 intracellular localization in mouse and human choroid plexus epithelium with development. Int. J. Dev. Biol. 2005, 49, 859–865.
- Erb, U.; Schwerk, C.; Schroten, H.; Karremann, M. Review of functional in vitro models of the blood-cerebrospinal fluid barrier in leukaemia research. J. Neurosci. Methods 2020, 329, 108478.
- Madea, B.; Musshoff, F. Postmortem biochemistry. Forensic Sci. Int. 2007, 165, 165–171.
- Sauer, S.W.; Opp, S.; Mahringer, A.; Kamiński, M.M.; Thiel, C.; Okun, J.G.; Fricker, G.; Morath, M.A.; Kölker, S. Glutaric aciduria type I and methylmalonic aciduria: Simulation of cerebral import and export of accumulating neurotoxic dicarboxylic acids in in vitro models of the blood-brain barrier and the choroid plexus. Biochim. Biophys. Acta 2010, 1802, 552–560.
- Kuplennik, N.; Lang, K.; Steinfeld, R.; Sosnik, A. Folate receptor α-modified nanoparticles for targeting of the central nervous system. ACS Appl. Mater. Interfaces 2019, 11, 39633–39647.
- Strazielle, N.; Ghersi-Egea, J.-F. In vitro investigation of the blood-cerebrospinal fluid barrier properties: Primary cultures and immortalized cell lines of the choroidal epithelium. In The Blood-Cerebrospinal Fluid Barrier; Zheng, W., Chodobski, A., Eds.; CRC Press: Boca Raton, FL, USA, 2005.
- Lallai, V.; Ahmed, A.; Fowler, C.D. Method for primary epithelial cell culture from the rat choroid plexus. Bio Protoc. 2020, 10, e3532.
- Wang, X.; Li, G.J.; Zheng, W. Upregulation of DMT1 expression in choroidal epithelia of the blood—CSF barrier following manganese exposure in vitro. Brain Res. 2006, 1097, 1–10.
- Kitazawa, T.; Hosoya, K.; Watanabe, M.; Takashima, T.; Ohtsuki, S.; Takanaga, H.; Ueda, M.; Yanai, N.; Obinata, M.; Terasaki, T. Characterization of the amino acid transport of new immortalized choroid plexus epithelial cell lines: A novel in vitro system for investigating transport functions at the blood-cerebrospinal fluid barrier. Pharm. Res. 2001, 18, 16–22.
- Zheng, W.; Zhao, Q. Establishment and characterization of an immortalized Z310 choroidal epithelial cell line from murine choroid plexus. Brain Res. 2002, 958, 371–380.
- Kläs, J.; Wolburg, H.; Terasaki, T.; Fricker, G.; Reichel, V. Characterization of immortalized choroid plexus epithelial cell lines for studies of transport processes across the blood-cerebrospinal fluid barrier. Cerebrospinal Fluid Res. 2010, 7, 11.
- Fujiyoshi, M.; Ohtsuki, S.; Hori, S.; Tachikawa, M.; Terasaki, T. 24S-hydroxycholesterol induces cholesterol release from choroid plexus epithelial cells in an apical- and apoE isoform-dependent manner concomitantly with the induction of ABCA1 and ABCG1 expression. J. Neurochem. 2007, 100, 968–978.
- Li, G.J.; Zhao, Q.; Zheng, W. Alteration at translational but not transcriptional level of transferrin receptor expression following manganese exposure at the blood-CSF barrier in vitro. Toxicol. Appl. Pharmacol. 2005, 205, 188–200.
- Tachikawa, M.; Fujinawa, J.; Takahashi, M.; Kasai, Y.; Fukaya, M.; Sakai, K.; Yamazaki, M.; Tomi, M.; Watanabe, M.; Sakimura, K.; et al. Expression and possible role of creatine transporter in the brain and at the blood-cerebrospinal fluid barrier as a transporting protein of guanidinoacetate, an endogenous convulsant. J. Neurochem. 2008, 107, 768–778.
- Shi, L.Z.; Li, G.J.; Wang, S.; Zheng, W. Use of Z310 cells as an in vitro blood-cerebrospinal fluid barrier model: Tight junction proteins and transport properties. Toxicol. In Vitro 2008, 22, 190–199.
- Terasaki, T.; Hosoya, K. Conditionally immortalized cell lines as a new in vitro model for the study of barrier functions. Biol. Pharm. Bull. 2001, 24, 111–118.
- Enjoji, M.; Iwaki, T.; Hara, H.; Sakai, H.; Nawata, H.; Watanabe, T. Establishment and characterization of choroid plexus carcinoma cell lines: Connection between choroid plexus and immune systems. Jpn. J. Cancer Res. 1996, 87, 893–899.
- Enjoji, M.; Iwaki, T.; Nawata, H.; Watanabe, T. IgH intronic enhancer element HE2 (μB) functions as a cis-activator in choroid plexus cells at the cellular level as well as in transgenic mice. J. Neurochem. 1995, 64, 961–966.
- Schroten, M.; Hanisch, F.G.; Quednau, N.; Stump, C.; Riebe, R.; Lenk, M.; Wolburg, H.; Tenenbaum, T.; Schwerk, C. A novel porcine in vitro model of the blood-cerebrospinal fluid barrier with strong barrier function. PLoS ONE 2012, 7, e39835.
- Lauer, A.N.; März, M.; Meyer, S.; Meurer, M.; de Buhr, N.; Borkowski, J.; Weiß, C.; Schroten, H.; Schwerk, C. Optimized cultivation of porcine choroid plexus epithelial cells, a blood-cerebrospinal fluid barrier model, for studying granulocyte transmigration. Lab. Investig. 2019, 99, 1245–1255.
- Thörnwall, M.; Chhajlani, V.; Le Grevès, P.; Nyberg, F. Detection of growth hormone receptor mRNA in an ovine choroid plexus epithelium cell line. Biochem. Biophys. Res. Commun. 1995, 217, 349–353.
- Oelschlegel, A.M.; Geissen, M.; Lenk, M.; Riebe, R.; Angermann, M.; Schatzl, H.; Groschup, M.H. A bovine cell line that can be infected by natural sheep scrapie prions. PLoS ONE 2015, 10, e0117154.
- Albert, O.; Ancellin, N.; Preisser, L.; Morel, A.; Corman, B. Serotonin, bradykinin and endothelin signalling in a sheep choroid plexus cell line. Life Sci. 1999, 64, 859–867.
- Merino, B.; Díez-Fernández, C.; Ruiz-Gayo, M.; Somoza, B. Choroid plexus epithelial cells co-express the long and short form of the leptin receptor. Neurosci. Lett. 2006, 393, 269–272.
- Angelova, K.; Fralish, G.B.; Puett, D.; Narayan, P. Identification of conventional and novel endothelin receptors in sheep choroid plexus cells. Mol. Cell. Biochem. 1996, 159, 65–72.
- Dickinson, K.E.; Baska, R.A.; Cohen, R.B.; Bryson, C.C.; Smith, M.A.; Schroeder, K.; Lodge, N.J. Identification of P1075 binding sites and P1075-activated K+ currents in ovine choroid plexus cells. Eur. J. Pharmacol. 1998, 345, 97–101.
- Callegari, E.; Malhotra, B.; Bungay, P.J.; Webster, R.; Fenner, K.S.; Kempshall, S.; LaPerle, J.L.; Michel, M.C.; Kay, G.G. A comprehensive non-clinical evaluation of the CNS penetration potential of antimuscarinic agents for the treatment of overactive bladder. Br. J. Clin. Pharmacol. 2011, 72, 235–246.
- Fischer, H.; Senn, C.; Ullah, M.; Cantrill, C.; Schuler, F.; Yu, L. Calculation of an apical efflux ratio from P-plycoprotein (P-gp) in vitro transport experiments shows an improved correlation with in vivo cerebrospinal fluid measurements in rats: Impact on P-gp screening and compound optimization. J. Pharmacol. Exp. Ther. 2021, 376, 322–329.
- Braun, A.; Hämmerle, S.; Suda, K.; Rothen-Rutishauser, B.; Günthert, M.; Krämer, S.D.; Wunderli-Allenspach, H. Cell cultures as tools in biopharmacy. Eur. J. Pharm. Sci. 2000, 11 (Suppl. 2), S51–S60.
- Fischer, H.; Ullah, M.; de la Cruz, C.C.; Hunsaker, T.; Senn, C.; Wirz, T.; Wagner, B.; Draganov, D.; Vazvaei, F.; Donzelli, M.; et al. Entrectinib, a TRK/ROS1 inhibitor with anti-CNS tumor activity: Differentiation from other inhibitors in its class due to weak interaction with P-glycoprotein. Neuro Oncol. 2020, 22, 819–829.
- Mangas-Sanjuan, V.; González-Álvarez, I.; González-Álvarez, M.; Casabó, V.G.; Bermejo, M. Innovative in vitro method to predict rate and extent of drug delivery to the brain across the blood-brain barrier. Mol. Pharm. 2013, 10, 3822–3831.
- Terasaki, T.; Ohtsuki, S.; Hori, S.; Takanaga, H.; Nakashima, E.; Hosoya, K. New approaches to in vitro models of blood-brain barrier drug transport. Drug Discov. Today 2003, 8, 944–954.
- Feng, B.; Doran, A.C.; Di, L.; West, M.A.; Osgood, S.M.; Mancuso, J.Y.; Shaffer, C.L.; Tremaine, L.; Liras, J. Prediction of human brain penetration of P-glycoprotein and breast cancer resistance protein substrates using in vitro transporter studies and animal models. J. Pharm. Sci. 2018, 107, 2225–2235.
- Tachibana, K.; Hashimoto, Y.; Shirakura, K.; Okada, Y.; Hirayama, R.; Iwashita, Y.; Nishino, I.; Ago, Y.; Takeda, H.; Kuniyasu, H.; et al. Safety and efficacy of an anti-claudin-5 monoclonal antibody to increase blood-brain barrier permeability for drug delivery to the brain in a non-human primate. J. Control. Release 2021, 336, 105–111.
- Nicolas, J.M.; Chanteux, H.; Nicolaï, J.; Brouta, F.; Viot, D.; Rosseels, M.L.; Gillent, E.; Bonnaillie, P.; Mathy, F.X.; Long, J.; et al. Role of P-glycoprotein in the brain disposition of seletalisib: Evaluation of the potential for drug-drug interactions. Eur. J. Pharm. Sci. 2020, 142, 105122.
- Ye, D.; Dawson, K.A.; Lynch, I. A TEM protocol for quality assurance of in vitro cellular barrier models and its application to the assessment of nanoparticle transport mechanisms across barriers. Analyst 2015, 140, 83–97.
- Srinivasan, B.; Kolli, A.R.; Esch, M.B.; Abaci, H.E.; Shuler, M.L.; Hickman, J.J. TEER measurement techniques for in vitro barrier model systems. J. Lab. Autom. 2015, 20, 107–126.
- Vigh, J.P.; Kincses, A.; Ozgür, B.; Walter, F.R.; Santa-Maria, A.R.; Valkai, S.; Vastag, M.; Neuhaus, W.; Brodin, B.; Dér, A.; et al. Transendothelial electrical resistance measurement across the blood-brain barrier: A critical review of methods. Micromachines 2021, 12, 685.
- Nag, S. Blood-brain barrier permeability using tracers and immunohistochemistry. Methods Mol. Med. 2003, 89, 133–144.
- Neal, E.H.; Shi, Y.; Lippmann, E.S. In vitro blood-brain barrier functional assays in a human iPSC-based model. In Cell Culture Techniques; Aschner, M., Costa, L., Eds.; Springer: New York, NY, USA, 2019; pp. 1–15.
- Nitz, T.; Eisenblätter, T.; Haselbach, M.; Galla, H.-J. Recent advances in the development of cell culture models for the blood-brain- and blood-CSF-barrier. In Blood—Brain Barrier: Drug Delivery and Brain Pathology; Kobiler, D., Lustig, S., Shapira, S., Eds.; Springer: Boston, MA, USA, 2001; pp. 45–62.
- Wegener, J.; Hakvoort, A.; Galla, H.J. Barrier function of porcine choroid plexus epithelial cells is modulated by cAMP-dependent pathways in vitro. Brain Res. 2000, 853, 115–124.
- Duffey, M.E.; Hainau, B.; Ho, S.; Bentzel, C.J. Regulation of epithelial tight junction permeability by cyclic AMP. Nature 1981, 294, 451–453.
- Tenenbaum, T.; Matalon, D.; Adam, R.; Seibt, A.; Wewer, C.; Schwerk, C.; Galla, H.J.; Schroten, H. Dexamethasone prevents alteration of tight junction-associated proteins and barrier function in porcine choroid plexus epithelial cells after infection with Streptococcus suis in vitro. Brain Res. 2008, 1229, 1–17.
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A novel Transwell blood brain barrier model using primary human cells. Front. Cell. Neurosci. 2019, 13, 230.
- Shi, L.Z.; Zheng, W. Establishment of an in vitro brain barrier epithelial transport system for pharmacological and toxicological study. Brain Res. 2005, 1057, 37–48.
- Förster, C.; Burek, M.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Drenckhahn, D. Differential effects of hydrocortisone and TNFalpha on tight junction proteins in an in vitro model of the human blood-brain barrier. J. Physiol. 2008, 586, 1937–1949.
- Siegal, T. Strategies for increasing drug delivery to the brain. In Blood—Brain Barrier: Drug Delivery and Brain Pathology; Kobiler, D., Lustig, S., Shapira, S., Eds.; Springer: Boston, MA, USA, 2001; pp. 251–271.
- Southwell, B.R.; Duan, W.; Alcorn, D.; Brack, C.; Richardson, S.J.; Köhrle, J.; Schreiber, G. Thyroxine transport to the brain: Role of protein synthesis by the choroid plexus. Endocrinology 1993, 133, 2116–2126.
- Peiser, C.; McGregor, G.P.; Lang, R.E. Binding and internalization of leptin by porcine choroid plexus cells in culture. Neurosci. Lett. 2000, 283, 209–212.
- Tachikawa, M.; Kasai, Y.; Takahashi, M.; Fujinawa, J.; Kitaichi, K.; Terasaki, T.; Hosoya, K. The blood-cerebrospinal fluid barrier is a major pathway of cerebral creatinine clearance: Involvement of transporter-mediated process. J. Neurochem. 2008, 107, 432–442.
- Duarte, A.C.; Rosado, T.; Costa, A.R.; Santos, J.; Gallardo, E.; Quintela, T.; Ishikawa, H.; Schwerk, C.; Schroten, H.; Gonçalves, I.; et al. The bitter taste receptor TAS2R14 regulates resveratrol transport across the human blood-cerebrospinal fluid barrier. Biochem. Pharmacol. 2020, 177, 113953.
- Baird, A.; Eliceiri, B.P.; Gonzalez, A.M.; Johanson, C.E.; Leadbeater, W.; Stopa, E.G. Targeting the choroid plexus-CSF-brain nexus using peptides identified by phage display. Methods Mol. Biol. 2011, 686, 483–498.
- Vercauteren, D.; Vandenbroucke, R.E.; Jones, A.T.; Rejman, J.; Demeester, J.; De Smedt, S.C.; Sanders, N.N.; Braeckmans, K. The use of inhibitors to study endocytic pathways of gene carriers: Optimization and pitfalls. Mol. Ther. 2010, 18, 561–569.
- Gomez-Zepeda, D.; Taghi, M.; Scherrmann, J.M.; Decleves, X.; Menet, M.C. ABC transporters at the blood-brain interfaces, their study models, and drug delivery implications in gliomas. Pharmaceutics 2019, 12, 20.
- Kaur, M.; Badhan, R.K. Phytoestrogens modulate breast cancer resistance protein expression and function at the blood-cerebrospinal fluid barrier. J. Pharm. Pharm. Sci. 2015, 18, 132–154.
- Sanders-Bush, E.; Breeding, M. Choroid plexus epithelial cells in primary culture: A model of 5HT1C receptor activation by hallucinogenic drugs. Psychopharmacology 1991, 105, 340–346.
- Strazielle, N.; Ghersi-Egea, J.-F. In vitro models of the blood-cerebrospinal fluid barrier and their use in neurotoxicological research. In Cell Culture Techniques. Neuromethods; Aschner, M., Suñol, C., Bal-Price, A., Eds.; Humana Press: Totowa, NJ, USA, 2011; Volume 56.
- Dragunow, M. Meningeal and choroid plexus cells—Novel drug targets for CNS disorders. Brain Res. 2013, 1501, 32–55.
- Johanson, C.; Johanson, N. Merging transport data for choroid plexus with blood-brain barrier to model CNS homeostasis and disease more effectively. CNS Neurol. Disord. Drug Targets 2016, 15, 1151–1180.
- Marques, F.; Sousa, J.C.; Brito, M.A.; Pahnke, J.; Santos, C.; Correia-Neves, M.; Palha, J.A. The choroid plexus in health and in disease: Dialogues into and out of the brain. Neurobiol. Dis. 2017, 107, 32–40.
- Safaee, M.; Oh, M.C.; Bloch, O.; Sun, M.Z.; Kaur, G.; Auguste, K.I.; Tihan, T.; Parsa, A.T. Choroid plexus papillomas: Advances in molecular biology and understanding of tumorigenesis. Neuro Oncol. 2013, 15, 255–267.
This entry is offline, you can click here to edit this entry!
