One grand challenge for studying plant biotic and abiotic stress responses is to optimize plant growth and plasticity under variable environmental constraints, which in the long run benefits agricultural production. However, efforts in promoting plant immunity are often accompanied by compromised morphological “syndromes” such as growth retardation, sterility, and reduced yield. Such a trade-off is dictated by complex signaling driven by secondary messengers and phytohormones. Salicylic acid (SA) is a well-known phytohormone essential for basal immunity and systemic acquired resistance. Interestingly, updates suggest that external environmental cues, nutrient status, developmental stages, primary metabolism, and breeding strategies attribute an additional layer of control over SA-dependent signaling, and, hence, plant performance against pathogens.
- plant immunity
- salicylic acid
- phytohormones
- growth–defense trade-off
1. Introduction
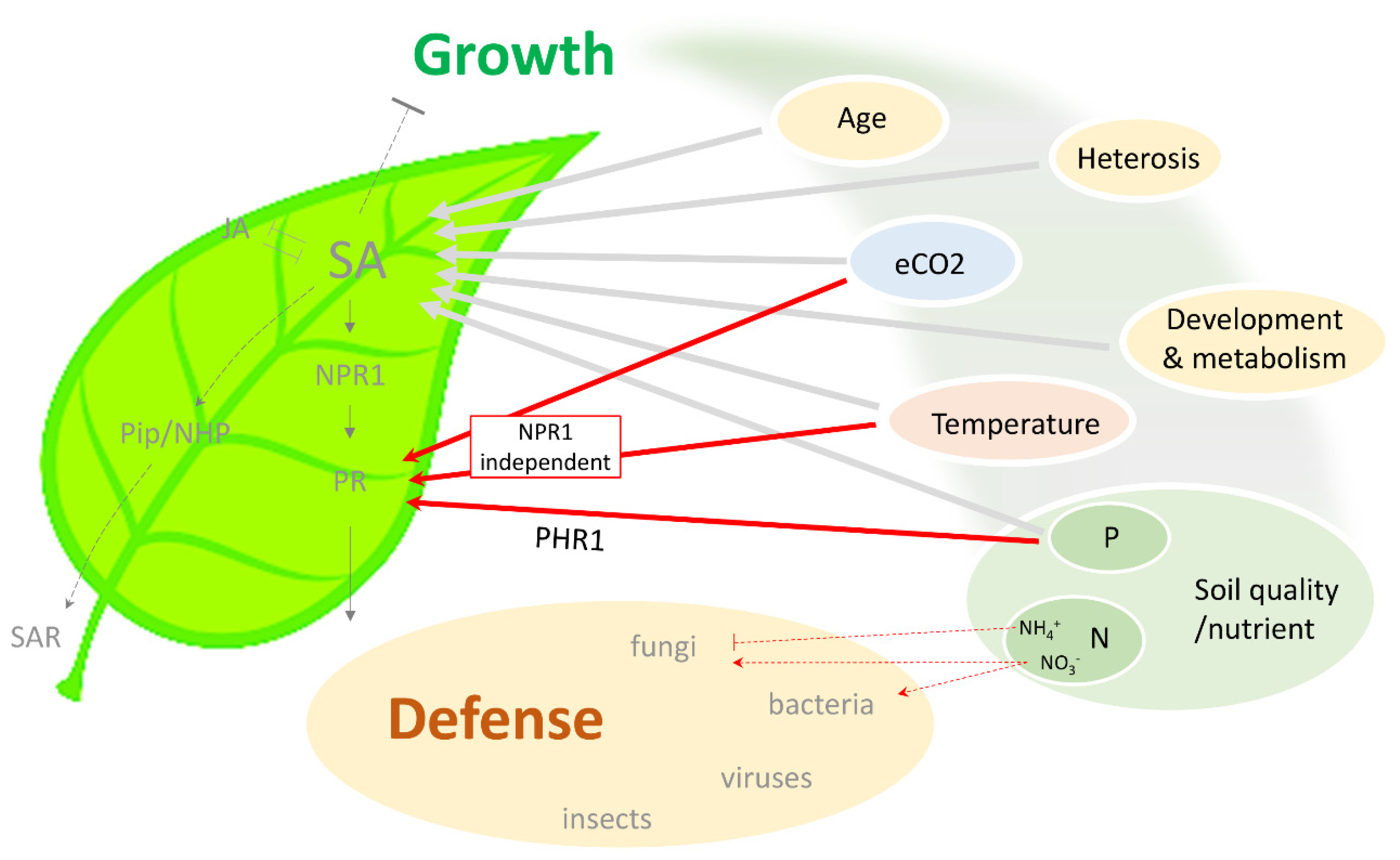
2. SA Biosynthesis, Perception, and Signaling in Defense and Growth
3. Environmental Conditions Modulate SA Biosynthesis and SA Signaling
3.1. Temperature
3.2. Atmospheric CO2
3.3. Nutrient Status Modulates SA-Dependent Immunity
This entry is adapted from the peer-reviewed paper 10.3390/cells11192985
References
- Huot, B.; Yao, J.; Montgomery, B.L.; He, S.Y. Growth–Defense Tradeoffs in Plants: A Balancing Act to Optimize Fitness. Mol. Plant 2014, 7, 1267–1287.
- Kim, J.H.; Hilleary, R.; Seroka, A.; He, S.Y. Crops of the future: Building a climate-resilient plant immune system. Curr. Opin. Plant Biol. 2021, 60, 101997.
- Prasch, C.M.; Sonnewald, U. Simultaneous Application of Heat, Drought, and Virus to Arabidopsis Plants Reveals Significant Shifts in Signaling Networks. Plant Physiol. 2013, 162, 1849–1866.
- Zhang, H.; Sonnewald, U. Differences and commonalities of plant responses to single and combined stresses. Plant J. 2017, 90, 839–855.
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiol. 2004, 134, 1683–1696.
- Rasmussen, S.; Barah, P.; Suarez-Rodriguez, M.C.; Bressendorff, S.; Friis, P.; Costantino, P.; Bones, A.M.; Nielsen, H.B.; Mundy, J. Transcriptome Responses to Combinations of Stresses in Arabidopsis. Plant Physiol. 2013, 161, 1783–1794.
- Ding, P.; Ding, Y. Stories of Salicylic Acid: A Plant Defense Hormone. Trends Plant Sci. 2020, 25, 549–565.
- Peng, Y.; Yang, J.; Li, X.; Zhang, Y. Salicylic Acid: Biosynthesis and Signaling. Annu. Rev. Plant Biol. 2021, 72, 761–791.
- Zhang, L.; Zhang, F.; Melotto, M.; Yao, J.; He, S.Y. Jasmonate signaling and manipulation by pathogens and insects. J. Exp. Bot. 2017, 68, 1371–1385.
- Rekhter, D.; Lüdke, D.; Ding, Y.; Feussner, K.; Zienkiewicz, K.; Lipka, V.; Wiermer, M.; Zhang, Y.; Feussner, I. Isochorismate-derived biosynthesis of the plant stress hormone salicylic acid. Science 2019, 365, 498.
- Yamasaki, K.; Motomura, Y.; Yagi, Y.; Nomura, H.; Kikuchi, S.; Nakai, M.; Shiina, T. Chloroplast envelope localization of EDS5, an essential factor for salicylic acid biosynthesis in Arabidopsis thaliana. Plant Signal. Behav. 2013, 8, e23603.
- Serrano, M.; Wang, B.; Aryal, B.; Garcion, C.; Abou-Mansour, E.; Heck, S.; Geisler, M.; Mauch, F.; Nawrath, C.; Métraux, J.-P. Export of Salicylic Acid from the Chloroplast Requires the Multidrug and Toxin Extrusion-Like Transporter EDS5. Plant Physiol. 2013, 162, 1815–1821.
- Torrens-Spence, M.P.; Bobokalonova, A.; Carballo, V.; Glinkerman, C.M.; Pluskal, T.; Shen, A.; Weng, J.-K. PBS3 and EPS1 Complete Salicylic Acid Biosynthesis from Isochorismate in Arabidopsis. Mol. Plant 2019, 12, 1577–1586.
- Huang, J.; Gu, M.; Lai, Z.; Fan, B.; Shi, K.; Zhou, Y.-H.; Yu, J.-Q.; Chen, Z. Functional Analysis of the Arabidopsis PAL Gene Family in Plant Growth, Development, and Response to Environmental Stress. Plant Physiol. 2010, 153, 1526–1538.
- Ding, Y.; Sun, T.; Ao, K.; Peng, Y.; Zhang, Y.; Li, X.; Zhang, Y. Opposite Roles of Salicylic Acid Receptors NPR1 and NPR3/NPR4 in Transcriptional Regulation of Plant Immunity. Cell 2018, 173, 1454–1467.
- Wu, Y.; Zhang, D.; Chu, J.Y.; Boyle, P.; Wang, Y.; Brindle, I.D.; De Luca, V.; Després, C. The Arabidopsis NPR1 Protein Is a Receptor for the Plant Defense Hormone Salicylic Acid. Cell Rep. 2012, 1, 639–647.
- Cao, H.; Glazebrook, J.; Clarke, J.D.; Volko, S.; Dong, X. The Arabidopsis NPR1 Gene That Controls Systemic Acquired Resistance Encodes a Novel Protein Containing Ankyrin Repeats. Cell 1997, 88, 57–63.
- Rochon, A.; Boyle, P.; Wignes, T.; Fobert, P.R.; Després, C. The Coactivator Function of Arabidopsis NPR1 Requires the Core of Its BTB/POZ Domain and the Oxidation of C-Terminal Cysteines. Plant Cell 2006, 18, 3670–3685.
- Rivas-San Vicente, M.; Plasencia, J. Salicylic acid beyond defence: Its role in plant growth and development. J. Exp. Bot. 2011, 62, 3321–3338.
- Gutiérrez-Coronado, M.A.; Trejo-López, C.; Larqué-Saavedra, A. Effects of salicylic acid on the growth of roots and shoots in soybean. Plant Physiol. Biochem. 1998, 36, 563–565.
- Shakirova, F.M.; Sakhabutdinova, A.R.; Bezrukova, M.V.; Fatkhutdinova, R.A.; Fatkhutdinova, D.R. Changes in the hormonal status of wheat seedlings induced by salicylic acid and salinity. Plant Sci. 2003, 164, 317–322.
- Gunes, A.; Inal, A.; Alpaslan, M.; Eraslan, F.; Bagci, E.G.; Cicek, N. Salicylic acid induced changes on some physiological parameters symptomatic for oxidative stress and mineral nutrition in maize (Zea mays L.) grown under salinity. J. Plant Physiol. 2007, 164, 728–736.
- Kovácik, J.; Grúz, J.; Backor, M.; Strnad, M.; Repcák, M. Salicylic acid-induced changes to growth and phenolic metabolism in Matricaria chamomilla plants. Plant Cell Rep. 2009, 28, 135–143.
- van Wersch, R.; Li, X.; Zhang, Y. Mighty Dwarfs: Arabidopsis Autoimmune Mutants and Their Usages in Genetic Dissection of Plant Immunity. Front. Plant Sci. 2016, 7, 1717.
- Abreu, M.E.; Munné-Bosch, S. Salicylic acid deficiency in NahG transgenic lines and sid2 mutants increases seed yield in the annual plant Arabidopsis thaliana. J. Exp. Bot. 2009, 60, 1261–1271.
- Samuel, G. Some Experiments On Inoculating Methods With Plant Viruses, And On Local Lesions. Ann. Appl. Biol. 1931, 18, 494–507.
- Dropkin, V.H. The necrotic reaction of tomatoes and other hosts resistant to Meloidogyne: Reversal by temperature. Phytopathology 1969, 59, 1632–1637.
- Kim, Y.; Park, S.; Gilmour, S.J.; Thomashow, M.F. Roles of CAMTA transcription factors and salicylic acid in configuring the low-temperature transcriptome and freezing tolerance of Arabidopsis. Plant J. 2013, 75, 364–376.
- Wu, Z.; Han, S.; Zhou, H.; Tuang, Z.K.; Wang, Y.; Jin, Y.; Shi, H.; Yang, W. Cold stress activates disease resistance in Arabidopsis thaliana through a salicylic acid dependent pathway. Plant Cell Environ. 2019, 42, 2645–2663.
- Li, Z.; Liu, H.M.; Ding, Z.H.; Yan, J.P.; Yu, H.Y.; Pan, R.H.; Hu, J.; Guan, Y.J.; Hua, J. Low Temperature Enhances Plant Immunity via Salicylic Acid Pathway Genes That Are Repressed by Ethylene. Plant Physiol. 2020, 182, 626–639.
- Whitham, S.; McCormick, S.; Baker, B. The N gene of tobacco confers resistance to tobacco mosaic virus in transgenic tomato. Proc. Natl. Acad. Sci. USA 1996, 93, 8776–8781.
- Anfoka, G.; Moshe, A.; Fridman, L.; Amrani, L.; Rotem, O.; Kolot, M.; Zeidan, M.; Czosnek, H.; Gorovits, R. Tomato yellow leaf curl virus infection mitigates the heat stress response of plants grown at high temperatures. Sci. Rep. 2016, 6, 19715.
- Makarova, S.; Makhotenko, A.; Spechenkova, N.; Love, A.J.; Kalinina, N.O.; Taliansky, M. Interactive Responses of Potato (Solanum tuberosum L.) Plants to Heat Stress and Infection With Potato Virus Y. Front. Microbiol. 2018, 9, 2582.
- Wang, Y.; Bao, Z.; Zhu, Y.; Hua, J. Analysis of Temperature Modulation of Plant Defense Against Biotrophic Microbes. Mol. Plant Microbe Interact. 2009, 22, 498–506.
- Zhu, Y.; Qian, W.; Hua, J. Temperature Modulates Plant Defense Responses through NB-LRR Proteins. PLoS Pathog. 2010, 6, e1000844.
- Noctor, G.; Mhamdi, A. Climate Change, CO2, and Defense: The Metabolic, Redox, and Signaling Perspectives. Trends Plant Sci. 2017, 22, 857–870.
- Niu, Y.; Ahammed, G.J.; Tang, C.; Guo, L.; Yu, J. Physiological and Transcriptome Responses to Combinations of Elevated CO2 and Magnesium in Arabidopsis thaliana. PLoS ONE 2016, 11, e0149301.
- Kane, K.; Dahal, K.P.; Badawi, M.A.; Houde, M.; Hüner, N.P.A.; Sarhan, F. Long-Term Growth Under Elevated CO2 Suppresses Biotic Stress Genes in Non-Acclimated, But Not Cold-Acclimated Winter Wheat. Plant Cell Physiol. 2013, 54, 1751–1768.
- Williams, A.; Petriacq, P.; Schwarzenbacher, R.E.; Beerling, D.J.; Ton, J. Mechanisms of glacial-to-future atmospheric CO2 effects on plant immunity. New Phytol. 2018, 218, 752–761.
- Mhamdi, A.; Noctor, G. High CO2 Primes Plant Biotic Stress Defences through Redox-Linked Pathways. Plant Physiol. 2016, 172, 929–942.
- Zhang, S.; Li, X.; Sun, Z.; Shao, S.; Hu, L.; Ye, M.; Zhou, Y.; Xia, X.; Yu, J.; Shi, K. Antagonism between phytohormone signalling underlies the variation in disease susceptibility of tomato plants under elevated CO2. J. Exp. Bot. 2015, 66, 1951–1963.
- Vaughan, M.M.; Huffaker, A.; Schmelz, E.A.; Dafoe, N.J.; Christensen, S.; Sims, J.; Martins, V.F.; Swerbilow, J.A.Y.; Romero, M.; Alborn, H.T.; et al. Effects of elevated on maize defence against mycotoxigenic Fusarium verticillioides. Plant Cell Environ. 2014, 37, 2691–2706.
- Váry, Z.; Mullins, E.; McElwain, J.C.; Doohan, F.M. The severity of wheat diseases increases when plants and pathogens are acclimatized to elevated carbon dioxide. Glob. Chang. Biol. 2015, 21, 2661–2669.
- Zhou, Y.L.; Van Leeuwen, S.K.; Pieterse, C.M.J.; Bakker, P.; Van Wees, S.C.M. Effect of atmospheric CO2 on plant defense against leaf and root pathogens of Arabidopsis. Eur. J. Plant Pathol. 2019, 154, 31–42.
- Eastburn, D.M.; Degennaro, M.M.; Delucia, E.H.; Dermody, O.; McElrone, A.J. Elevated atmospheric carbon dioxide and ozone alter soybean diseases at SoyFACE. Glob. Change Biol. 2010, 16, 320–330.
- Eastburn, D.M.; McElrone, A.J.; Bilgin, D.D. Influence of atmospheric and climatic change on plant–pathogen interactions. Plant Pathol. 2011, 60, 54–69.
- Mur, L.A.J.; Simpson, C.; Kumari, A.; Gupta, A.K.; Gupta, K.J. Moving nitrogen to the centre of plant defence against pathogens. Ann. Bot. 2017, 119, 703–709.
- Chan, C.; Liao, Y.-Y.; Chiou, T.-J. The Impact of Phosphorus on Plant Immunity. Plant Cell Physiol. 2021, 62, 582–589.
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen uptake, assimilation and remobilization in plants: Challenges for sustainable and productive agriculture. Ann. Bot. 2010, 105, 1141–1157.
- Mus, F.; Crook Matthew, B.; Garcia, K.; Garcia Costas, A.; Geddes Barney, A.; Kouri Evangelia, D.; Paramasivan, P.; Ryu, M.-H.; Oldroyd Giles, E.D.; Poole Philip, S.; et al. Symbiotic Nitrogen Fixation and the Challenges to Its Extension to Nonlegumes. Appl. Environ. Microbiol. 2016, 82, 3698–3710.
- Hoffland, E.; Jeger, M.J.; van Beusichem, M.L. Effect of nitrogen supply rate on disease resistance in tomato depends on the pathogen. Plant Soil 2000, 218, 239–247.
- Huang, H.; Nguyen Thi Thu, T.; He, X.; Gravot, A.; Bernillon, S.; Ballini, E.; Morel, J.B. Increase of fungal pathogenicity and role of plant glutamine in nitrogen-induced susceptibility (NIS) to rice blast. Front. Plant Sci. 2017, 8, 265.
- Ballini, E.; Nguyen, T.T.T.; Morel, J.B. Diversity and genetics of Nitrogen-Induced Susceptibility to the blast fungus in rice and wheat. Rice 2013, 6, 1–13.
- Hoffland, E.; van Beusichem, M.L.; Jeger, M.J. Nitrogen availability and susceptibility of tomato leaves to Botrytis cinerea. Plant Soil 1999, 210, 263–272.
- Solomon, P.S.; Oliver, R.P. The nitrogen content of the tomato leaf apoplast increases during infection by Cladosporium fulvum. Planta 2001, 213, 241–249.
- Pageau, K.; Reisdorf-Cren, M.; Morot-Gaudry, J.F.; Masclaux-Daubresse, C. The two senescence-related markers, GS1 (cytosolic glutamine synthetase) and GDH (glutamate dehydrogenase), involved in nitrogen mobilization, are differentially regulated during pathogen attack and by stress hormones and reactive oxygen species in Nicotiana tabacum L. leaves. J. Exp. Bot. 2006, 57, 547–557.
- Tavernier, V.; Cadiou, S.; Pageau, K.; Lauge, R.; Reisdorf-Cren, M.; Langin, T.; Masclaux-Daubresse, C. The plant nitrogen mobilization promoted by Colletotrichum lindemuthianum in Phaseolus leaves depends on fungus pathogenicity. J. Exp. Bot. 2007, 58, 3351–3360.
- López-Berges, M.S.; Rispail, N.; Prados-Rosales, R.C.; Di Pietro, A. A Nitrogen Response Pathway Regulates Virulence Functions in Fusarium oxysporum via the Protein Kinase TOR and the bZIP Protein MeaB. Plant Cell 2010, 22, 2459–2475.
