Hearing loss represents a significant handicap that gravely impacts the quality of life. Normal hearing function depends on the mechanical and physiological integrity of the middle and inner ear structures and their associated nervous system. The middle ear is composed of the tympanic membrane and the ossicles: malleus, incus and stapes. Accordingly, middle ear pathologies primarily involve the mechanical compromise of the eardrum or its associated ossicles due to, for example, an infection, a fluid accumulation or trauma. Corticosteroid therapy has been mainly effective in stopping, decreasing or correcting an auditory impairment in numerous other etiologies of hearing loss, a steroid therapy has also been encouraged in the field of surgical inner ear interventions.
1. Cochlear Implantation and Glucocorticoid Therapy
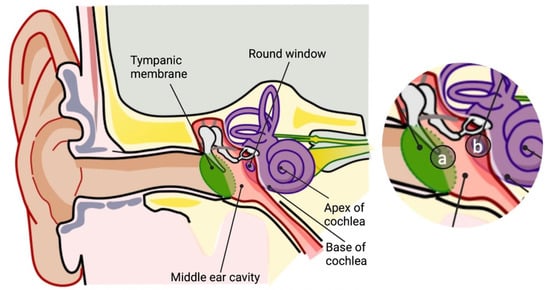
The now commonly practiced cochlear implantation procedure aims to restore the hearing capacity and improve the quality of life. This surgical procedure is fairly routine and is typically performed under general anesthesia. A small retro-auricular incision is made, the implant is then placed under the skin and the electrode is inserted into the cochlea through the round window (
Figure 1). Nevertheless, during the procedure, the delicate structures of the cochlea are exposed to a direct trauma [
25,
26]. Rapid inflammation ensues as the cascading molecular agents of the tissue injury rage onward, including an avalanche of ROS molecules; these events lead to hair cell death and a subsequent loss of the residual biological hearing capacity [
27,
28,
29,
30,
31]. Later on, fibrosis forms around the implanted electrode, a physiological phenomenon that occurs around practically any foreign object, and jeopardizes the conductive functionality of the device [
32]. The procedure of a cochlear implantation is, therefore, still evolving.
Figure 1. Routes of drug administration for inner ear treatments. Schematic representation of the anatomy of the ear. Inset, routes of direct drug injection: (a) intratympanic; (b) intracochlear through the round window (created with BioRender.com accessed on 9 September 2022).
Factors that benefit hearing preservation during a cochlear implantation have thus far included modified surgical practices (the angle and speed of the electrode insertion), flexible electrode arrays and the use of steroids [
33]. The standard of care most widely includes dexamethasone. Due to its potent anti-inflammatory and anti-proliferative properties, dexamethasone decreases post-operative inflammation and diminishes the fibrotic processes. There is, however, no consensus regarding the optimal timing, dosage and route of the steroidal administration as all methods to date have led to relatively satisfactory hearing preservation [
33,
34]. A glucocorticoid treatment in the context of a cochlear implantation can also be systemically administered. Several studies suggest that systemic glucocorticoids prior to a cochlear implantation improve hearing preservation. According to a prospective non-randomized study, patients treated with prolonged glucocorticoid regimens of combined oral and i.v. routes beginning 3 days before surgery had significantly superior hearing preservation outcomes 6 months post-surgery compared with the untreated control group. Positive hearing preservation differences were detected not only when compared with the control group, but also when compared with the standard treatment group, which began an i.v. treatment 30 min prior to surgery [
35]. When testing short-term single-dose regimens, a double-blind placebo-controlled trial found that patients undergoing a cochlear implantation and treated immediately before surgery with a single high dose of i.v. methylprednisolone had no difference in hearing preservation or electrode impedance compared with the placebo group [
36].
To avoid a systemic glucocorticoid exposure, the treatment may be introduced using an intratympanic delivery. Prospective clinical studies have shown that an intratympanic glucocorticoid therapy prior to an electrode insertion whilst repeatedly delivering glucocorticoids throughout the surgical procedure results in better post-operative hearing preservation [
37]. This was tested using intratympanic methylprednisone immediately after an intubation [
38] and intratympanic dexamethasone 15 min before an electrode insertion [
39]. Yet, there are several limitations to these studies; dexamethasone was applied together with hyaluronic acid, which minimizes the surgical trauma and seals the cochlea, thereby increasing the exposure to the drug. Accordingly, a double-blind placebo-controlled randomized trial showed that applying a polymeric sponge soaked with methylprednisolone onto the round window membrane during cochlear implantation surgery resulted in lower post-operative inner ear symptoms [
40]. Moreover, the glucocorticoid treatment group also exhibited a lower electrode impedance from the middle portion of the electrode array, possibly pointing towards a minimal accumulation of fibrosis around the electrode array. This also suggests that the drug reached the mid-range frequency zone of the cochlea, raising the possibility that a longer exposure time may allow glucocorticoids to reach more distally inside the cochlea.
Unlike in the case of a systemic or intratympanic glucocorticoid delivery, a direct intracochlear delivery places the drug at the site of damage, avoiding the reliance on drug diffusion. A clinical study by Passche et al. showed that a single intracochlear dose of triamcinolone prior to an electrode insertion lowered the electrode impedance [
41]. The difference in the electrode impedance levels between the treated and the control groups was maintained for up to 17 days post-operation, but was then lost. Prenzler et al. speculated that this significant, albeit short-term, effect was due to a low concentration of the drug and conducted a study using a 5-fold greater dose and a custom-designed catheter that reached deeper into the cochlea [
42]. The results confirmed that there was a significant difference between the high-dose group and the control group for up to an average of 39.53 days. When the high-dose and low-dose groups were compared, there was no significant difference at any point of the trial. Nevertheless, the significance between the low-dose group and the control group was lost at an earlier stage than with the high-dose group. It is important to note that when subdividing the electrode surfaces into basal, middle and apical contact sites, a significant difference was only observed in the basal and middle regions despite the deep insertion of the drug [
42].
Glucocorticoid delivery routes may also be combined. A retrospective cohort study compared an intratympanic treatment during surgery with an intratympanic treatment plus a systemic two-week oral glucocorticoid taper beginning three days prior to surgery [
43]. The results suggest that patients who received the combined regimen had a higher degree of hearing preservation. In that study, the three days of preoperative oral glucocorticoids may have resulted in higher perilymph glucocorticoid concentrations, augmenting the effect of the intratympanic glucocorticoid therapy. According to a randomized controlled trial, a combination of transtympanic glucocorticoids 24 h prior to surgery then local glucocorticoids during surgery achieved superior pure-tone average outcomes compared with either monotherapies [
44]. It is reasonable to propose that a preoperative glucocorticoid administration, whether systemic or local, may precondition the tissue and, together with a local intratympanic treatment during surgery, lead to improved outcomes. As the great majority of cochlear implants are planned ahead of time, the concept of preconditioning is worth exploring.
In an attempt to optimize the impact of dexamethasone within the inner ear compartment,
drug-eluting electrode arrays were developed. In this method, dexamethasone-loaded silicone rings are introduced between the stimulating channels that diffuse dexamethasone directly into the cochlea [
32]. The rationale behind these eluting electrode arrays is that they are able to release dexamethasone for an extended duration, possibly addressing a later-appearing fibrosis. Positive outcomes have been demonstrated in guinea pig and non-human primate models. In the guinea pig model, three dexamethasone doses were tested, resulting in significant dose-dependent positive outcomes; at two months post-surgery, the electrode impedance was still superior to the control electrode arrays [
45]. The immunostaining of the organ of Corti depicted preserved outer hair cells whereas the control group showed missing patches of both inner and outer hair cells. In a parallel study, the fibrosis around the electrode was diminished alongside an electrode impedance [
46]. These observations may offer an important development as they advance into clinical trials such as in the CI-DEX study (NCT04750642).
2. Stapedectomy and Glucocorticoid Therapy
A stapedectomy is performed to treat hearing loss caused by otosclerosis. Surgery is usually performed as an outpatient procedure under local or general anesthesia. Using an operating microscope, the surgeon elevates the eardrum and observes the stapes immobility as part of the diagnosis. Using delicate instruments and preferably a laser, the upper part of the stapes is removed. In order to re-establish the chain of soundwave transmissions, a small hole is made in the remaining part of the stapes, the footplate. A prothesis is then inserted into the hole and attached to the second bone in the ossicle chain, the incus. Inevitably, during a stapedectomy, the barrier to the cochlear labyrinth is breached, causing a trauma to the vessels of the perilymphatic space and manifesting as serous labyrinthitis and/or a reparative granuloma [
47,
48,
49]. The incidence of a partial or complete loss of sensorineural hearing due to this procedure is 0.6% and 3%, respectively [
47]. The perioperative administration of corticosteroids is not under a consensus as it has been advocated by a few [
50,
51,
52,
53] and disputed by others [
54].
3. Perilymphatic Fistula and Glucocorticoid Therapy
A perilymphatic fistula is an improper connection between the perilymph-filled inner ear and either the cavity of the middle ear, the mastoid bone or the cerebral cavity. It may be trauma-induced or idiopathic and caused cochlear and vestibular symptoms in both cases. There are essentially two types of treatment for a perilymphatic fistula: conservative and surgical. Conservative management is favored based on the etiology and severity of the condition; it tries to avoid any measure that might raise intracranial pressure or inner ear pressure and focuses on intratympanic or systemic steroids in cases of an acute decompensation [
55,
56].
4. Post-Surgical Facial Paralysis and Glucocorticoid Therapy
The facial nerve may become paralyzed following ear surgeries, particularly those performed in the middle ear. Such a direct iatrogenic paralysis of the facial nerve, which can occur during or just after surgery, has been the subject of extensive research. Delayed facial paralysis is defined as occurring at least 48 h after surgery and occurs after 0.2 to 1.9% of middle ear surgeries [
57]. The mechanism behind this latent phenomenon can be one of three: facial canal dehiscence (the partial or total separation of previously approximated wound edges due to a failed wound healing process); a neural edema; or herpes virus reactivation. An edema, for example, is implied in 17% of stapes surgery procedures [
58]. It leads to a compression of the nerve fibers and a disruption of the blood supply causing facial nerve weakness; the process peaks on the fifth day post-surgery and almost completely recedes within 14 days [
57]. By default, a steroid therapy is applied with the aim of, at the very least, reducing the edema.
This entry is adapted from the peer-reviewed paper 10.3390/app12189359