Gut microbiota is a complex system that starts to take shape early in life. Several factors influence the rise of microbial gut colonization, such as term and mode of delivery, exposure to antibiotics, maternal diet, presence of siblings and family members, pets, genetics, local environment, and geographical location. Breastfeeding, complementary feeding, and later dietary patterns during infancy and toddlerhood are major players in the proper development of microbial communities. Nonetheless, if dysbiosis occurs, gut microbiota may remain impaired throughout life, leading to deleterious consequences, such as greater predisposition to non-communicable diseases, more susceptible immune system and altered gut–brain axis.
- gut microbiota
- infants
- standard diets
- special diets
1. Introduction
2. Gut Microbiota in Infants and Toddlers under Standard Diets
2.1. Breast Milk vs. Formula Milk and Gut Microbiota
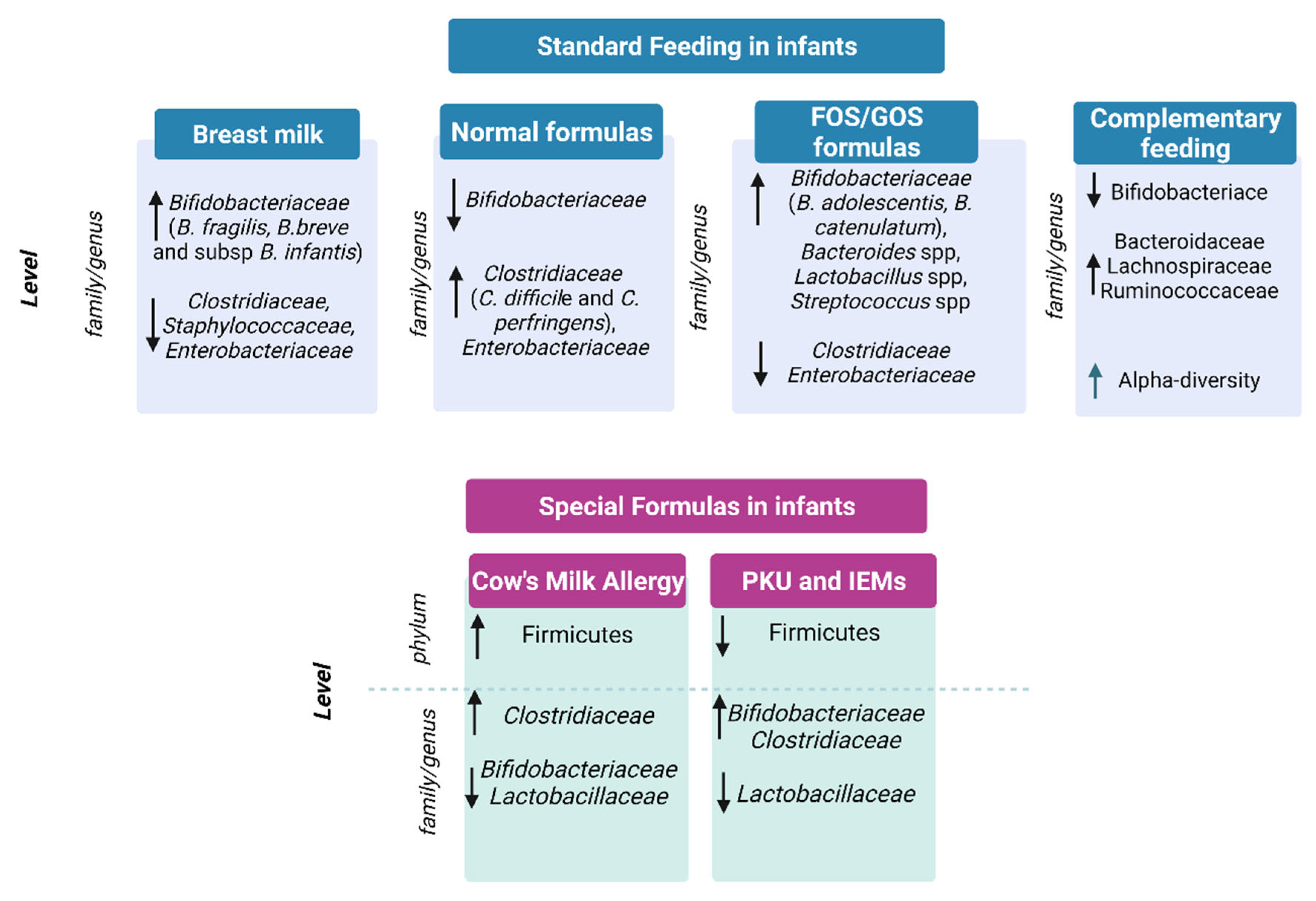
2.2. Gut Microbiota and Complementary Feeding
3. Gut Microbiota in Infants under Special Diets
3.1. Special Formulas for the Treatment of Cow’s Milk Allergy
3.2. Special Formula for Functional Gastrointestinal Symptoms
3.3. Phenylketonuria and Inborn Errors of Metabolism Formulas
This entry is adapted from the peer-reviewed paper 10.3390/nu14153198
References
- Passos, M.D.C.F.; Moraes-Filho, J.P. Intestinal Microbiota in Digestive Diseases. Arq. Gastroenterol. 2017, 54, 255–262.
- Gomaa, E.Z. Human Gut Microbiota/Microbiome in Health and Diseases: A Review. Antonie Van Leeuwenhoek 2020, 113, 2019–2040.
- Alonso, V.R.; Guarner, F. Linking the Gut Microbiota to Human Health. Br. J. Nutr. 2013, 109, S21–S26.
- Jethwani, P.; Grover, K. Gut Microbiota in Health and Diseases—A Review. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1586–1599.
- Laursen, M.F.; Bahl, M.I.; Licht, T.R. Settlers of Our Inner Surface—Factors Shaping the Gut Microbiota from Birth to Toddlerhood. FEMS Microbiol. Rev. 2021, 45, fuab001.
- Arrieta, M.-C.; Stiemsma, L.T.; Amenyogbe, N.; Brown, E.M.; Finlay, B. The Intestinal Microbiome in Early Life: Health and Disease. Front. Immunol. 2014, 5, 427.
- Bergström, A.; Skov, T.H.; Bahl, M.I.; Roager, H.M.; Christensen, L.B.; Ejlerskov, K.T.; Mølgaard, C.; Michaelsen, K.F.; Licht, T.R. Establishment of Intestinal Microbiota during Early Life: A Longitudinal, Explorative Study of a Large Cohort of Danish Infants. Appl. Environ. Microbiol. 2014, 80, 2889–2900.
- Laursen, M.F. Gut Microbiota Development: Influence of Diet from Infancy to Toddlerhood. Ann. Nutr. Metab. 2021, 77, 1–14.
- Chong, C.; Bloomfield, F.; O’Sullivan, J. Factors Affecting Gastrointestinal Microbiome Development in Neonates. Nutrients 2018, 10, 274.
- Bittinger, K.; Zhao, C.; Li, Y.; Ford, E.; Friedman, E.S.; Ni, J.; Kulkarni, C.V.; Cai, J.; Tian, Y.; Liu, Q.; et al. Bacterial Colonization Reprograms the Neonatal Gut Metabolome. Nat. Microbiol. 2020, 5, 838–847.
- Obermajer, T.; Grabnar, I.; Benedik, E.; Tušar, T.; Pikel, T.R.; Mis, N.F.; Matijašić, B.B.; Rogelj, I. Microbes in Infant Gut Development: Placing Abundance Within Environmental, Clinical and Growth Parameters. Sci. Rep. 2017, 7, 11230.
- Jian, C.; Luukkonen, P.; Yki-Järvinen, H.; Salonen, A.; Korpela, K. Quantitative PCR Provides a Simple and Accessible Method for Quantitative Microbiota Profiling. PLoS ONE 2020, 15, e0227285.
- Laursen, M.F.; Bahl, M.I.; Michaelsen, K.F.; Licht, T.R. First Foods and Gut Microbes. Front. Microbiol. 2017, 8, 356.
- World Health Organization, WHO. Available online: https://www.who.int/health-topics/com-plementary-feeding#tab=tab_2 (accessed on 1 May 2022).
- Simpson, H.L.; Campbell, B.J. Review Article: Dietary Fibre-Microbiota Interactions. Aliment. Pharmacol. Ther. 2015, 42, 158–179.
- Koh, A.; De Vadder, F.; Kovatcheva-Datchary, P.; Bäckhed, F. From Dietary Fiber to Host Physiology: Short-Chain Fatty Acids as Key Bacterial Metabolites. Cell 2016, 165, 1332–1345.
- Davis, J.C.C.; Totten, S.M.; Huang, J.O.; Nagshbandi, S.; Kirmiz, N.; Garrido, D.A.; Lewis, Z.T.; Wu, L.D.; Smilowitz, J.T.; German, J.B.; et al. Identification of Oligosaccharides in Feces of Breast-Fed Infants and Their Correlation with the Gut Microbial Community. Mol. Cell. Proteom. 2016, 15, 2987–3002.
- WHO. Protecting, Promoting, and Supporting Breastfeeding in Facilities Providing Maternity and Newborn Services: The Revised Baby-Friendly Hospital Initiative 2018; WHO: Geneva, Switzerland, 2018.
- Section on Breastfeeding; Eidelman, A.I.; Schanler, R.J.; Johnston, M.; Landers, S.; Noble, L.; Szucs, K.; Viehmann, L. Breastfeeding and the Use of Human Milk. Pediatrics 2012, 129, e827–e841.
- Agostoni, C.; Braegger, C.; Decsi, T.; Kolacek, S.; Koletzko, B.; Michaelsen, K.F.; Mihatsch, W.; Moreno, L.A.; Puntis, J.; Shamir, R.; et al. Breast-Feeding: A Commentary by the ESPGHAN Committee on Nutrition. J. Pediatr. Gastroenterol. Nutr. 2009, 49, 112–125.
- Sakanaka, M.; Gotoh, A.; Yoshida, K.; Odamaki, T.; Koguchi, H.; Xiao, J.; Kitaoka, M.; Katayama, T. Varied Pathways of Infant Gut-Associated Bifidobacterium to Assimilate Human Milk Oligosaccharides: Prevalence of the Gene Set and Its Correlation with Bifidobacteria-Rich Microbiota Formation. Nutrients 2019, 12, 71.
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria Can Protect from Enteropathogenic Infection through Production of Acetate. Nature 2011, 469, 543–547.
- Henrick, B.M.; Rodriguez, L.; Lakshmikanth, T.; Pou, C.; Henckel, E.; Arzoomand, A.; Olin, A.; Wang, J.; Mikes, J.; Tan, Z.; et al. Bifidobacteria-Mediated Immune System Imprinting Early in Life. Cell 2021, 184, 3884–3898.e11.
- Stokholm, J.; Blaser, M.J.; Thorsen, J.; Rasmussen, M.A.; Waage, J.; Vinding, R.K.; Schoos, A.-M.M.; Kunøe, A.; Fink, N.R.; Chawes, B.L.; et al. Maturation of the Gut Microbiome and Risk of Asthma in Childhood. Nat. Commun. 2018, 9, 141.
- Vatanen, T.; Kostic, A.D.; d’Hennezel, E.; Siljander, H.; Franzosa, E.A.; Yassour, M.; Kolde, R.; Vlamakis, H.; Arthur, T.D.; Hämäläinen, A.-M.; et al. Variation in Microbiome LPS Immunogenicity Contributes to Autoimmunity in Humans. Cell 2016, 165, 842–853.
- Alcon-Giner, C.; Dalby, M.J.; Caim, S.; Ketskemety, J.; Shaw, A.; Sim, K.; Lawson, M.A.E.; Kiu, R.; Leclaire, C.; Chalklen, L.; et al. Microbiota Supplementation with Bifidobacterium and Lactobacillus Modifies the Preterm Infant Gut Microbiota and Metabolome: An Observational Study. Cell Rep. Med. 2020, 1, 100077.
- Cerdó, T.; Ruíz, A.; Suárez, A.; Campoy, C. Probiotic, Prebiotic, and Brain Development. Nutrients 2017, 9, 1247.
- Ma, J.; Li, Z.; Zhang, W. Comparison of the Gut Microbiota in Healthy Infants With Different Delivery Modes and Feeding Types: A Cohort Study. Front. Microbiol. 2022, 13, 868227.
- Bäckhed, F.; Roswall, J.; Peng, Y.; Feng, Q.; Jia, H.; Kovatcheva-Datchary, P.; Li, Y.; Xia, Y.; Xie, H.; Zhong, H.; et al. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. Cell Host Microbe 2015, 17, 690–703.
- Berger, B.; Porta, N.; Foata, F.; Grathwohl, D.; Delley, M.; Moine, D.; Charpagne, A.; Siegwald, L.; Descombes, P.; Alliet, P.; et al. Linking Human Milk Oligosaccharides, Infant Fecal Community Types, and Later Risk to Require Antibiotics. mBio 2020, 11, e03196-19.
- Salminen, S.; Stahl, B.; Vinderola, G.; Szajewska, H. Infant Formula Supplemented with Biotics: Current Knowledge and Future Perspectives. Nutrients 2020, 12, 1952.
- Pärnänen, K.; Hultman, J.; Satokari, R.; Rautava, S.; Lamendella, R.; Wright, J.; McLimans, C.J.; Kelleher, S.L.; Virta, M. Formula alters preterm infant gut microbiota and increases its antibiotic resistance load. bioRxiv 2019, 782441.
- He, X.; Parenti, M.; Grip, T.; Lönnerdal, B.; Timby, N.; Domellöf, M.; Hernell, O.; Slupsky, C.M. Fecal Microbiome and Metabolome of Infants Fed Bovine MFGM Supplemented Formula or Standard Formula with Breast-Fed Infants as Reference: A Randomized Controlled Trial. Sci. Rep. 2019, 9, 11589.
- Chow, J.; Panasevich, M.R.; Alexander, D.; Vester Boler, B.M.; Rossoni Serao, M.C.; Faber, T.A.; Bauer, L.L.; Fahey, G.C. Fecal Metabolomics of Healthy Breast-Fed versus Formula-Fed Infants before and during In Vitro Batch Culture Fermentation. J. Proteome Res. 2014, 13, 2534–2542.
- Ehrlich, A.M.; Pacheco, A.R.; Henrick, B.M.; Taft, D.; Xu, G.; Huda, M.N.; Mishchuk, D.; Goodson, M.L.; Slupsky, C.; Barile, D.; et al. Indole-3-Lactic Acid Associated with Bifidobacterium-Dominated Microbiota Significantly Decreases Inflammation in Intestinal Epithelial Cells. BMC Microbiol. 2020, 20, 357.
- Meng, D.; Sommella, E.; Salviati, E.; Campiglia, P.; Ganguli, K.; Djebali, K.; Zhu, W.; Walker, W.A. Indole-3-Lactic Acid, a Metabolite of Tryptophan, Secreted by Bifidobacterium longum Subspecies Infantis Is Anti-Inflammatory in the Immature Intestine. Pediatr. Res. 2020, 88, 209–217.
- Henrick, B.M.; Chew, S.; Casaburi, G.; Brown, H.K.; Frese, S.A.; Zhou, Y.; Underwood, M.A.; Smilowitz, J.T. Colonization by B. infantis EVC001 Modulates Enteric Inflammation in Exclusively Breastfed Infants. Pediatr. Res. 2019, 86, 749–757.
- Shaw, A.G.; Cornwell, E.; Sim, K.; Thrower, H.; Scott, H.; Brown, J.C.S.; Dixon, R.A.; Kroll, J.S. Dynamics of Toxigenic Clostridium perfringens Colonisation in a Cohort of Prematurely Born Neonatal Infants. BMC Pediatr. 2020, 20, 75.
- Kuiper, G.-A.; van Prehn, J.; Ang, W.; Kneepkens, F.; van der Schoor, S.; de Meij, T. Clostridium difficile Infections in Young Infants: Case Presentations and Literature Review. IDCases 2017, 10, 7–11.
- Saraf, M.K.; Piccolo, B.D.; Bowlin, A.K.; Mercer, K.E.; LeRoith, T.; Chintapalli, S.V.; Shankar, K.; Badger, T.M.; Yeruva, L. Formula Diet Driven Microbiota Shifts Tryptophan Metabolism from Serotonin to Tryptamine in Neonatal Porcine Colon. Microbiome 2017, 5, 77.
- Fernández-Reina, A.; Urdiales, J.; Sánchez-Jiménez, F. What We Know and What We Need to Know about Aromatic and Cationic Biogenic Amines in the Gastrointestinal Tract. Foods 2018, 7, 145.
- Isolauri, E.; Rautava, S.; Salminen, S.; Collado, M.C. Early-Life Nutrition and Microbiome Development. In Nestlé Nutrition Institute Workshop Series; Donovan, S.M., German, J.B., Lönnerdal, B., Lucas, A., Eds.; S. Karger AG: Berlin, Germany, 2019; Volume 90, pp. 151–162. ISBN 978-3-318-06340-0.
- Differding, M.K.; Benjamin-Neelon, S.E.; Hoyo, C.; Østbye, T.; Mueller, N.T. Timing of Complementary Feeding Is Associated with Gut Microbiota Diversity and Composition and Short Chain Fatty Acid Concentrations over the First Year of Life. BMC Microbiol. 2020, 20, 56.
- Leong, C.; Haszard, J.J.; Lawley, B.; Otal, A.; Taylor, R.W.; Szymlek-Gay, E.A.; Fleming, E.A.; Daniels, L.; Fangupo, L.J.; Tannock, G.W.; et al. Mediation Analysis as a Means of Identifying Dietary Components That Differentially Affect the Fecal Microbiota of Infants Weaned by Modified Baby-Led and Traditional Approaches. Appl. Environ. Microbiol. 2018, 84, e00914-18.
- Fallani, M.; Amarri, S.; Uusijarvi, A.; Adam, R.; Khanna, S.; Aguilera, M.; Gil, A.; Vieites, J.M.; Norin, E.; Young, D.; et al. Determinants of the Human Infant Intestinal Microbiota after the Introduction of First Complementary Foods in Infant Samples from Five European Centres. Microbiology 2011, 157, 1385–1392.
- Laursen, M.F.; Andersen, L.B.B.; Michaelsen, K.F.; Mølgaard, C.; Trolle, E.; Bahl, M.I.; Licht, T.R. Infant Gut Microbiota Development Is Driven by Transition to Family Foods Independent of Maternal Obesity. mSphere 2016, 1, e00069-15.
- Tang, M.; Frank, D.; Hendricks, A.; Ir, D.; Krebs, N. Protein Intake During Early Complementary Feeding Affects the Gut Microbiota in U.S. Formula-Fed Infants (FS04-03-19). Curr. Dev. Nutr. 2019, 3, nzz048.FS04-03-19.
- Sicherer, S.H. Epidemiology of Food Allergy. J. Allergy Clin. Immunol. 2011, 127, 594–602.
- Muraro, A.; Werfel, T.; Hoffmann-Sommergruber, K.; Roberts, G.; Beyer, K.; Bindslev-Jensen, C.; Cardona, V.; Dubois, A.; duToit, G.; Eigenmann, P.; et al. EAACI Food Allergy and Anaphylaxis Guidelines: Diagnosis and Management of Food Allergy. Allergy 2014, 69, 1008–1025.
- D’Auria, E.; Salvatore, S.; Acunzo, M.; Peroni, D.; Pendezza, E.; Di Profio, E.; Fiore, G.; Zuccotti, G.V.; Verduci, E. Hydrolysed Formulas in the Management of Cow’s Milk Allergy: New Insights, Pitfalls and Tips. Nutrients 2021, 13, 2762.
- Koletzko, S.; Niggemann, B.; Arato, A.; Dias, J.A.; Heuschkel, R.; Husby, S.; Mearin, M.L.; Papadopoulou, A.; Ruemmele, F.M.; Staiano, A.; et al. Diagnostic Approach and Management of Cow’s-Milk Protein Allergy in Infants and Children: ESPGHAN GI Committee Practical Guidelines. J. Pediatr. Gastroenterol. Nutr. 2012, 55, 221–229.
- von Berg, A. The Role of Hydrolysates for Allergy Prevention—Pro. Pediatr. Allergy Immunol. 2013, 24, 720–723.
- D’Auria, E.; Salvatore, S.; Pozzi, E.; Mantegazza, C.; Sartorio, M.U.A.; Pensabene, L.; Baldassarre, M.E.; Agosti, M.; Vandenplas, Y.; Zuccotti, G. Cow’s Milk Allergy: Immunomodulation by Dietary Intervention. Nutrients 2019, 11, 1399.
- Canani, R.B.; Nocerino, R.; Terrin, G.; Frediani, T.; Lucarelli, S.; Cosenza, L.; Passariello, A.; Leone, L.; Granata, V.; Di Costanzo, M.; et al. Formula Selection for Management of Children with Cow’s Milk Allergy Influences the Rate of Acquisition of Tolerance: A Prospective Multicenter Study. J. Pediatr. 2013, 163, 771–777.e1.
- Katz, Y.; Rajuan, N.; Goldberg, M.R.; Eisenberg, E.; Heyman, E.; Cohen, A.; Leshno, M. Early Exposure to Cow’s Milk Protein Is Protective against IgE-Mediated Cow’s Milk Protein Allergy. J. Allergy Clin. Immunol. 2010, 126, 77–82.e1.
- Paparo, L.; Picariello, G.; Bruno, C.; Pisapia, L.; Canale, V.; Sarracino, A.; Nocerino, R.; Carucci, L.; Cosenza, L.; Cozzolino, T.; et al. Tolerogenic Effect Elicited by Protein Fraction Derived From Different Formulas for Dietary Treatment of Cow’s Milk Allergy in Human Cells. Front. Immunol. 2020, 11, 604075.
- Tordesillas, L.; Berin, M.C. Mechanisms of Oral Tolerance. Clin. Rev. Allergy Immunol. 2018, 55, 107–117.
- Stefka, A.T.; Feehley, T.; Tripathi, P.; Qiu, J.; McCoy, K.; Mazmanian, S.K.; Tjota, M.Y.; Seo, G.-Y.; Cao, S.; Theriault, B.R.; et al. Commensal Bacteria Protect against Food Allergen Sensitization. Proc. Natl. Acad. Sci. USA 2014, 111, 13145–13150.
- Canani, R.B.; Gilbert, J.A.; Nagler, C.R. The Role of the Commensal Microbiota in the Regulation of Tolerance to Dietary Allergens. Curr. Opin. Allergy Clin. Immunol. 2015, 15, 243–249.
- Arpaia, N.; Campbell, C.; Fan, X.; Dikiy, S.; van der Veeken, J.; deRoos, P.; Liu, H.; Cross, J.R.; Pfeffer, K.; Coffer, P.J.; et al. Metabolites Produced by Commensal Bacteria Promote Peripheral Regulatory T-Cell Generation. Nature 2013, 504, 451–455.
- Paparo, L.; Nocerino, R.; Di Scala, C.; Della Gatta, G.; Di Costanzo, M.; Buono, A.; Bruno, C.; Canani, R.B. Targeting Food Allergy with Probiotics. Probiotics Child Gastrointest. Health 2019, 1125, 57–68.
- Canani, R.B.; Sangwan, N.; Stefka, A.T.; Nocerino, R.; Paparo, L.; Aitoro, R.; Calignano, A.; Khan, A.A.; Gilbert, J.A.; Nagler, C.R. Lactobacillus rhamnosus GG-Supplemented Formula Expands Butyrate-Producing Bacterial Strains in Food Allergic Infants. ISME J. 2016, 10, 742–750.
- Nocerino, R.; Bedogni, G.; Carucci, L.; Cosenza, L.; Cozzolino, T.; Paparo, L.; Palazzo, S.; Riva, L.; Verduci, E.; Canani, R.B. The Impact of Formula Choice for the Management of Pediatric Cow’s Milk Allergy on the Occurrence of Other Allergic Manifestations: The Atopic March Cohort Study. J. Pediatr. 2021, 232, 183–191.e3.
- Tan, J.; McKenzie, C.; Potamitis, M.; Thorburn, A.N.; Mackay, C.R.; Macia, L. The Role of Short-Chain Fatty Acids in Health and Disease. Adv. Immunol. 2014, 121, 91–119.
- Rivera-Chávez, F.; Zhang, L.F.; Faber, F.; Lopez, C.A.; Byndloss, M.X.; Olsan, E.E.; Xu, G.; Velazquez, E.M.; Lebrilla, C.B.; Winter, S.E.; et al. Depletion of Butyrate-Producing Clostridia from the Gut Microbiota Drives an Aerobic Luminal Expansion of Salmonella. Cell Host Microbe 2016, 19, 443–454.
- Tan, J.; McKenzie, C.; Vuillermin, P.J.; Goverse, G.; Vinuesa, C.G.; Mebius, R.E.; Macia, L.; Mackay, C.R. Dietary Fiber and Bacterial SCFA Enhance Oral Tolerance and Protect against Food Allergy through Diverse Cellular Pathways. Cell Rep. 2016, 15, 2809–2824.
- Furusawa, Y.; Obata, Y.; Fukuda, S.; Endo, T.A.; Nakato, G.; Takahashi, D.; Nakanishi, Y.; Uetake, C.; Kato, K.; Kato, T.; et al. Commensal Microbe-Derived Butyrate Induces the Differentiation of Colonic Regulatory T Cells. Nature 2013, 504, 446–450.
- Fujimura, K.E.; Lynch, S.V. Microbiota in Allergy and Asthma and the Emerging Relationship with the Gut Microbiome. Cell Host Microbe 2015, 17, 592–602.
- Perezabad, L.; López-Abente, J.; Alonso-Lebrero, E.; Seoane, E.; Pion, M.; Correa-Rocha, R. The Establishment of Cow’s Milk Protein Allergy in Infants Is Related with a Deficit of Regulatory T Cells (Treg) and Vitamin D. Pediatr. Res. 2017, 81, 722–730.
- Shreffler, W.G.; Wanich, N.; Moloney, M.; Nowak-Wegrzyn, A.; Sampson, H.A. Association of Allergen-Specific Regulatory T Cells with the Onset of Clinical Tolerance to Milk Protein. J. Allergy Clin. Immunol. 2009, 123, 43–52.e7.
- Ruohtula, T.; de Goffau, M.C.; Nieminen, J.K.; Honkanen, J.; Siljander, H.; Hämäläinen, A.-M.; Peet, A.; Tillmann, V.; Ilonen, J.; Niemelä, O.; et al. Maturation of Gut Microbiota and Circulating Regulatory T Cells and Development of IgE Sensitization in Early Life. Front. Immunol. 2019, 10, 2494.
- Kok, C.R.; Brabec, B.; Chichlowski, M.; Harris, C.L.; Moore, N.; Wampler, J.L.; Vanderhoof, J.; Rose, D.; Hutkins, R. Stool Microbiome, PH and Short/Branched Chain Fatty Acids in Infants Receiving Extensively Hydrolyzed Formula, Amino Acid Formula, or Human Milk through Two Months of Age. BMC Microbiol. 2020, 20, 337.
- Berni Canani, R.; De Filippis, F.; Nocerino, R.; Paparo, L.; Di Scala, C.; Cosenza, L.; Della Gatta, G.; Calignano, A.; De Caro, C.; Laiola, M.; et al. Gut Microbiota Composition and Butyrate Production in Children Affected by Non-IgE-Mediated Cow’s Milk Allergy. Sci. Rep. 2018, 8, 12500.
- Smilowitz, J.T.; Lebrilla, C.B.; Mills, D.A.; German, J.B.; Freeman, S.L. Breast Milk Oligosaccharides: Structure-Function Relationships in the Neonate. Annu. Rev. Nutr. 2014, 34, 143–169.
- Puccio, G.; Alliet, P.; Cajozzo, C.; Janssens, E.; Corsello, G.; Sprenger, N.; Wernimont, S.; Egli, D.; Gosoniu, L.; Steenhout, P. Effects of Infant Formula with Human Milk Oligosaccharides on Growth and Morbidity: A Randomized Multicenter Trial. J. Pediatr. Gastroenterol. Nutr. 2017, 64, 624–631.
- Nowak-Wegrzyn, A.; Czerkies, L.; Reyes, K.; Collins, B.; Heine, R.G. Confirmed Hypoallergenicity of a Novel Whey-Based Extensively Hydrolyzed Infant Formula Containing Two Human Milk Oligosaccharides. Nutrients 2019, 11, 1447.
- Hilvo, M. Maternal Elimination Diet and Symptoms of Cow’s Milk Allergy in Breastfed Infants. JAMA Pediatr. 2021, 175, 425–426.
- Vandenplas, Y.; Dupont, C.; Eigenmann, P.; Host, A.; Kuitunen, M.; Ribes-Koninckx, C.; Shah, N.; Shamir, R.; Staiano, A.; Szajewska, H.; et al. A Workshop Report on the Development of the Cow’s Milk-Related Symptom Score Awareness Tool for Young Children. Acta Paediatr. 2015, 104, 334–339.
- Vandenplas, Y.; Steenhout, P.; Planoudis, Y.; Grathwohl, D. Althera Study Group Treating Cow’s Milk Protein Allergy: A Double-Blind Randomized Trial Comparing Two Extensively Hydrolysed Formulas with Probiotics. Acta Paediatr. 2013, 102, 990–998.
- Salvatore, S.; Agosti, M.; Baldassarre, M.E.; D’Auria, E.; Pensabene, L.; Nosetti, L.; Vandenplas, Y. Cow’s Milk Allergy or Gastroesophageal Reflux Disease-Can We Solve the Dilemma in Infants? Nutrients 2021, 13, 297.
- Vandenplas, Y.; Salvatore, S. Infant Formula with Partially Hydrolyzed Proteins in Functional Gastrointestinal Disorders. Nestle Nutr. Inst. Workshop Ser. 2016, 86, 29–37.
- Dupont, C.; Bradatan, E.; Soulaines, P.; Nocerino, R.; Berni-Canani, R. Tolerance and Growth in Children with Cow’s Milk Allergy Fed a Thickened Extensively Hydrolyzed Casein-Based Formula. BMC Pediatr. 2016, 16, 96.
- MacDonald, A.; Cochrane, B.; Wopereis, H.; Loveridge, N. Specific Prebiotics in a Formula for Infants with Phenylketonuria. Mol. Genet. Metab. 2011, 104, S55–S59.
