Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Oncology
Polyphenols represent a structural class of mainly natural organic chemicals that contain multiple phenol structural units. Their antineoplastic effects have been demonstrated in various studies when they were tested on numerous cancer lines and some in in vivo models.
- reactive oxygen species
- oxidative stress
- carcinogenesis
- polyphenols
1. Introduction
Polyphenols represent a structural class of mainly natural organic chemicals that contain multiple phenol structural units. They can be classified into various subgroups depending on the number of phenol rings and based on structural elements that hold these rings together. Accordingly, the main classes of polyphenols are phenolic acids, flavonoids, stilbenes, and lignans. Cancer development is a complex process defined by three major stages: initiation, promotion, and progression. Initiation is a fast and irreversible step that can be generated by the uptake of, or exposure to, a carcinogenic agent, followed by its interaction with chromatin, leading to mutation or epigenetic modification [1].
Cancer patients first started to focus on natural products in their fight against the disease, mostly because of the numerous severe side effects and secondary toxicity induced by most conventional therapies [2]. At the same time, the pharmaceutical industry is testing new natural products that can be used in cancer treatment [2].
The advancements in cancer therapy were hampered by the appearance of drug resistance, high treatment costs, and increased reports of secondary toxicity, despite all the efforts made to raise awareness, early diagnosis, and new therapeutic interventions. In addition, known side effects commonly associated with the majority of chemotherapeutic drugs such as nausea, vomiting, headache, musculoskeletal pain, anorexia, gastritis, oral ulcers, diarrhea, constipation, alopecia, neuropathy, and so on, require additional therapies that further increase the treatment cost. The use of plants to fight cancer dates back several centuries, as reported in the ancient traditional folklore of Asia, Africa, and Europe. Various herb extracts and plant decoctions are considered to have the ability to prevent carcinogenesis, minimize tumor size, or remove cancer-related symptoms [2].
In this regard, natural products such as polyphenols may represent ideal alternatives, especially when administered with other drugs, where better efficacy and safety are necessary [3].
Classical anticancer therapies usually induce a hostile cellular environment, leading to organelle impairment around the tumor and the development of drug resistance. To overcome such adverse effects, studies have started to focus more on the use of bioactive anticancer compounds due to their multi-target specificity, selectivity, and their cyto-friendly nature [4].
Anticancer drugs can stop the unusual proliferation of malignant cells, encourage the apoptosis of cancer cells, and decrease metastasis, all by targeting various molecules and signaling pathways. Thus, the finding of the anticancer potential of natural polyphenols has become a great interest for pharmacists. Resveratrol, for instance, is one of the most studied polyphenols due to its great anticancer activity. Many studies have reported that the anticancer effects of resveratrol are effective on all cancer development stages (initiation, promotion, and progression) and can intervene in several signaling pathways such as the activation of pro-apoptosis pathways (p53, Bax/Bcl-2), the resistance of cell cycle (Cyclin, P21), the inhibition of metastasis and angiogenesis related pathways (VEGF, TGF-, MMP), and the regulation of inflammatory responses (NFB, MAPK). Other polyphenols have also showed anticancer potential such as curcumin, (−)-epigallocatechin-3-gallate (EGCG), genistein, etc. Meanwhile, many studies have reported that an unusual tumor microenvironment (i.e., acidic pH, increased ROS levels, hypoxic conditions) has a huge impact on increasing the genetic instability and appearance of drug resistance. Hence, it is very important to manage the normalization of the malignant tissue microenvironment. The well-known antioxidant nature of polyphenols can properly modulate the tumor microenvironment. Moreover, as already mentioned, polyphenols can act as both antioxidants and pro-oxidants. If the concentration increases, hydroxyphenoxyl radicals are able to react with a second radical and generate toxic quinone, which can lead to covalent DNA damage. Additionally, polyphenols possess great photothermal characteristics, which are excellent in photothermal therapy (i.e., polydopamine) [5].
Table 1 shows the main properties of some polyphenols (EGCG, curcumin, caffeic acid, and resveratrol).
| Polyphenol | Properties | Source | |
|---|---|---|---|
| EGCG | Antioxidant Antiproliferative Antiangiogenic Antimetastatic Proapoptotic |
Green tea |  |
| Curcumin | Antioxidant Anti-inflammatory Antiproliferative Antiangiogenic Antimetastatic Proapoptotic Chemo and radio sensitizer |
Turmeric |  |
| Caffeic acid | Antioxidant Anti-inflammatory Antineoplastic Antiviral |
Coffee beans |  |
| Resveratrol | Antioxidant Antiproliferative Antiangiogenic Proapoptotic Chemo and radio sensitizer |
Grape |  |
The numerous anticarcinogenic properties of polyphenols include their ability to suppress the formation of tumors, angiogenesis, metastasis, and inflammation as well as to trigger apoptosis. Additionally, they can control immune system responses and protect healthy cells from harmful free radicals. The majority of studies on the anticancer effects of polyphenols have been based on individual substances. For instance, resveratrol has been linked to a number of anti-cancer biological processes including the suppression of glucose uptake, metastasis, and the induction of apoptosis. It has been found that EGCG can control cancer cell growth, metastasis, angiogenesis, and other aspects of cancer evolution by altering several processes. Curcumin has also been shown to suppress cellular growth and angiogenesis, stop cell cycle progression in tumor cells, and trigger apoptosis in various cancer models (Figure 1) [8].
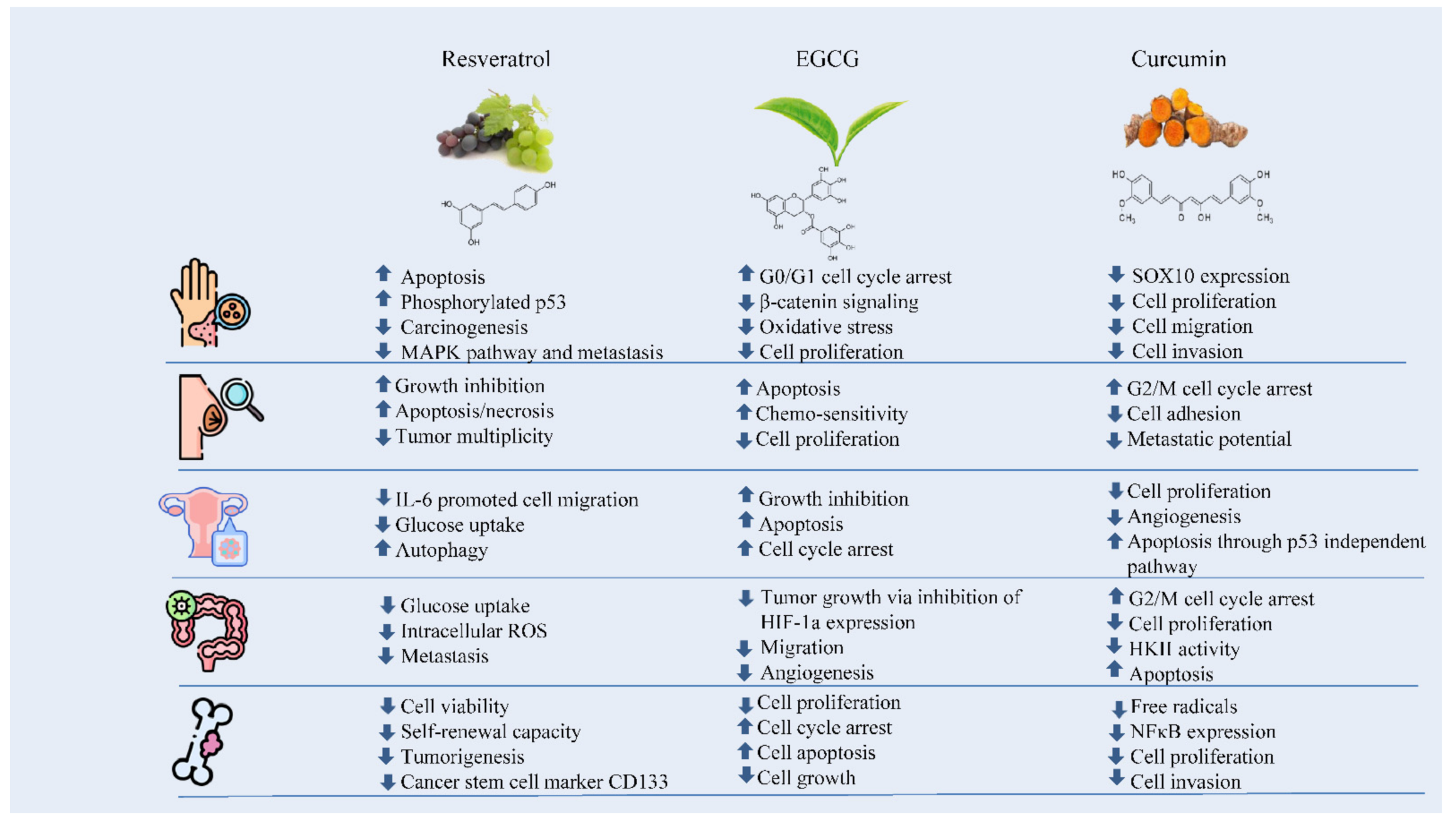
Figure 1. The effects of polyphenols against skin, breast, ovarian, colorectal, and bone cancers.
2. Skin Cancer
Skin cancers are the most frequent malignant neoplasm in humans, mostly in Caucasians. In the United States alone, more than two million people are annually diagnosed with non-melanoma and melanoma skin cancers. This means that the incidence of skin cancers is approximately equal to the combined incidence of cancers of all other organs. An important public health issue is represented by cutaneous cancers, which are a major health care expense. Even if sunscreens are used, they do not properly protect the skin against the damaging effects of solar ultraviolet radiation, which is an important cause of cutaneous malignancies. Therefore, it is highly necessary to design and develop functional therapeutic agents and more successful preventive approaches [9].
Skin possesses its own antioxidant protective mechanisms, which blocks some of the damaging effects of different carcinogens and environmental pollutants such as UV radiation, leading to the generation of oxygenated molecules called “free radicals”. However, when there is extensive exposure to these factors, the antioxidant capacity may be exceeded and become less efficient, causing premature aging, immunosuppression, and skin cancers. Prolonged carcinogen exposure can cause epidermal lipid peroxidation and unnecessary infiltration of the leukocytes into the skin. These can further cause the excessive production of hydrogen peroxide (H2O2), nitric oxide (NO), and other ROS, which leads to oxidative stress. Natural polyphenols protect cell constituents from oxidative damage by scavenging these free radicals [10].
The MAPK pathway consists of the extracellular signal-regulated kinase 1/2 (ERK 1/2), p38 proteins, and c-Jun-N-terminal-kinase (JNK). The activation of the MAP kinase pathway, mediated by the tyrosine kinase receptor, leads to the activation of transcription factor activator protein-1 (AP-1), which further activates the expression of MMPs. The p38 and JNK pathways are very important in increasing the expression of AP-1 and COX-2 mediated by UVA radiation and represent targets for skin cancer chemoprevention. The anticancer potential of polyphenols results from the inhibition of the MAPK pathway. For instance, black tea polyphenol and resveratrol reduced the expression of JNK, phosphorylated ERK 1/2, and p38 and enhanced apoptosis and phosphorylated p53 in the skin cancer cells, leading to the prevention of skin carcinogenesis. Resveratrol also inhibits cancer cell migration and metastasis through the inhibition of the MAPK pathway [11].
EGCG, a green tea polyphenol, has been reported to possess anti-carcinogenic properties on a number of skin tumor models, therefore, the focus has been on studying the molecular targets that are linked to its cytotoxicity against cancer cells. Cancer cell proliferation is accelerated by constant inflammation, which has been demonstrated to play an important role in β-catenin signaling activation. Due to recent observations of the fact that β-catenin is upregulated in skin cancer cells, it seems that the anti-skin carcinogenic properties of EGCG may be mainly mediated by its effect on β-catenin signaling [9].
Proliferation plays a huge role in cancer development and progression characterized by abnormal activity and the expression of cell cycle proteins. The cell cycle is the process of cell progression and division. Regulatory proteins involved in cell cycle are cyclines, CDK interacting proteins (CIPs) including p21, cyclin-dependent kinases (Cdks), kinase inhibitory proteins (KIPs) including p27, Cdk inhibitors (INKs) including p18, surviving and p53. Cancer is characterized by a poor functioning of these regulatory processes, which lead to unrestricted cell proliferation, and finally tumor growth and progression. EGCG has been reported to modulate the cell cycle through cell regulatory proteins, resulting in cell cycle arrest and decreased cellular proliferation. EGCG is able to arrest cells in the G0/G1 cell cycle phases, as long as its combination with other compounds has been demonstrated to induce G0/G1, G2/S, and G2/M cell cycle arrest in many cancer models [12].
Various polyphenols have been successfully used to inhibit TNF-α. For example, a polyphenol known as punicalagin, extracted from pomegranate, was used for the protection of human dermal fibroblasts from cell death caused by UV irradiation by downregulating NF-κB caspase-3 and upregulating the transition phase G0/G1 and the DNA repair process. EGCG and resveratrol are able to diminish UVB-induced ROS upregulation of TNF-α and IL-6, the levels of mRNA, and further inhibit NF-κB expression, leading to a general anti-inflammatory activity. Additionally, resveratrol inhibited the expression of transforming growth factor TGF-β2 caused by the skin cells’ exposure to UVB, which is linked to the blocking of TGF-β2/Smad-dependent and independent pathways [7].
Due to the benefits of polyphenols in in vitro and preclinical studies, clinical trials have also been conducted to reveal the protective activity of polyphenols in skin cancer. In a randomized clinical study, the antioxidative properties of mixtures made of tea polyphenols and milks were examined in 44 healthy subjects. It was observed that there was a reduced level of oxidative stress in the treatment group aside from the placebo group, which resulted in enhanced texture and integrity of thee dermis in young and aged subjects [7].
Moreover, it was observed that the A431 and SCC13 human skin cancer cell lines treated with EGCG presented a reduced cell viability and enhanced cell death, and as above-mentioned, these effects were due to the inactivation of β-catenin signaling. Additionally, EGCG seems to be able to exert a cytotoxic effect on malignant skin cells without a notable harm to healthy skin cells [9].
Studies have suggested that EGCG can also cause cell cycle arrest in A431 skin cancer cells by inhibiting Cip1/p21 with no other modifications in Kip1/p27, cyclin D1, and CDK2, but a decrease in CDK4 at low doses [12].
Aside from the topical application of polyphenols, dietary ingestion of grape seed extract has been reported to bring many benefits in avoiding DMBA-induced TPA promoted two-stage skin carcinogenesis, slowing down the malignant transformation of papillomas into carcinomas, diminishing DMBA-induced inflammatory hyperplasia and reducing the proportion of mice with codon 61 and Ha-ras oncogene mutations. Studies have suggested that topical as well as the dietary feeding of grape seed extracts (resveratrol, quercetin, catechin) had beneficial results in reducing DMBA-induced epidermal hyperplasia, inflammation, and proliferation. The concomitant oral and topical administration have been demonstrated to be more effective than separate use and lead to lower inflammation, oxidative stress, and mutations of Ha-ras in codon 61 [13].
Sticking to grape seed polyphenols, it has been demonstrated that SKH-1 hairless mice fed with grape seed proanthocyanidins presented a reduced tumor incidence, size, and proliferation in the complete stages (both initiation and promotion) of UVB-induced photocarcinogenesis. Moreover, the red grape seed extract showed great efficacy in preventing UVB-induced oxidative stress when applied directly on the skin by enhancing the levels of GSH and glutathione peroxidase through the inhibition of lipid peroxidation and nitric oxide generation. Additionally, grape seed polyphenols decreased the UVB-induced infiltration of proinflammatory leukocytes and reduced myeloperoxidase, prostaglandin, cyclooxygenase-2, cyclin D1, and proliferating cell nuclear antigen activities in skin tumors [13].
Toll-like receptor 4 (TRL4) seems to have a high importance in melanoma and tea polyphenols have a great anticancer activity. Thus, Chen et al. investigated the way that the tea polyphenols act on melanoma cells. In the study, tea polyphenols and lipopolysaccharides (LPS) were used to treat the B16F10 and A375 melanoma cell lines. Tea polyphenols reduced the proliferation, migration, and invasion capacity of cancer cells in a time and dosage dependent manner. It was observed that TRL4 was highly expressed in the skin cancer cells compared with the healthy skin cells. Tea polyphenols were able to inhibit TRL4 expression in both the stimulated and normal melanomas via the TRL4 antagonist LPS. TRL4 suppression can reduce cell function, therefore tea polyphenols have the ability to reduce melanoma growth in vivo [14].
3. Breast Cancer
Breast cancer represents one of the top causes of death in women worldwide. In a statistic from 2018 by the American Cancer Society, it was reported that around 30% of all new cancer cases in women were breast cancer and caused 40,920 deaths in the USA alone. It appears that one in eight women will suffer from breast cancer and the WHO reports show that the incidence will continue to raise. Around 80% of entire breast cancer cases diagnosed in postmenstrual females are estrogen receptor alpha positive, which means that they are very influenced by the presence of estrogen. Additionally, estrogen activity plays a highly important role in breast cancer prevention and therapy [15].
Resveratrol is a polyphenol usually found in grapes and red wine and has various health benefits. It has many great properties such as anticancer, neuro protective, anti-aging, antimicrobial, and anti-inflammatory. It has been reported that resveratrol is extremely beneficial against breast cancer mainly due to its ability to exert both anti-estrogenic and estrogenic effects (based on the concentration) and because it has a high affinity for estrogen receptors ERα and Erβ. At a concentration of 50 μM, it exerts anti-estrogenic effects in order to inhibit cell migration while, at a concentration of 5 μM, it exerts estrogenic effects by enhancing the invasion, migration, and development of lamellipodia on the ERα (−), ERβ (+) MDA-MB-231 breast cancer cell line. Lamellipodia is represented by some actin structures that can be observed at the leading edge of migrating cells, which are regulated by Rac. A total of 5 μM of resveratrol enhances Rac activity while 50 μM of resveratrol inhibits its activity in breast cancer cells. Concentration is also important in the case of Akt and MAPK. Increased concentrations reduce their activity while low concentrations have been reported to support proliferation in cancer cells and enhance Akt and MAPK activities, together with some other tumorigenic signaling proteins. Hence, the development and metastasis of breast cancer can be controlled based on the resveratrol dose [16].
Resveratrol is also a cycloocygenase-2 (COX-2) inhibitor, meaning that it has excellent anti-inflammatory properties. Considering all of the great properties exerted in human health, the addition of resveratrol in various mixtures has become very popular. COX inhibition and antioxidative activity are among the properties that participate in the commonly acknowledged anticancer and chemopreventive effects of resveratrol against a variety of malignancies including breast cancer. These mechanisms are able to keep the DNA away from oxidative damage and decrease prostaglandin-induced cancer cell proliferation. Moreover, it seems that resveratrol can also block other enzymes that participate in carcinogenesis and tumor progression such as ribonucleotide reductase, ribonuclease and human DNA ligase, RNA and DNA polymerases. Additionally, various in vitro studies have shown that resveratrol modulates gene expression and promotes the apoptosis of cancer cells via the downregulation of TP53, NF-κB, and Bcl-2, which are well-known transcription factors involved in tumor growth promoting gene activation [15].
One potential explanation regarding the double effect of resveratrol on Erα+ breast cancer cells is linked to the structural similarity of resveratrol with E2, which could mediate an interaction between resveratrol and both ERs, leading to estrogen-like effects and enhancing cancer cell proliferation. Although considering that the binding affinities of resveratrol to ERα and ERβ are as low as 0.0087 and 0.0102%, respectively, in comparison to original E2, there seem to be other mechanisms that are involved in the tumor growth inducing effects of resveratrol, especially at concentrations of less than 10 μM. A clinical study performed on 40 healthy female subjects showed that a daily high dose oral administration of 1.0, 2.5, or 5.0 g of resveratrol for 29 days led to high values of plasma levels far above the concentration needed to block the estrogen metabolism (0.62, 1.45, or 4.24 μM) [15].
Statistics show that HER-2 positive breast cancer is among the most aggressive subtypes and is responsible for approximately 30% of diagnosed cases, being associated with tumor invasiveness, low disease-free survival, and bad overall prognosis. Additionally, many patients have started to develop resistance to classical therapies. Thus, various current clinical trials are investigating new possible therapies. More and more studies have shown that polyphenols have great potential in breast cancer prevention and treatment, directly or indirectly via epigenetic regulation (i.e., micro RNAs) [17].
EGCG has been reported to be the only polyphenol that is found in plasma at high levels (77–90%) in free form. Its constant administration seems to help with breast cancer prevention by promoting apoptosis and reducing cell proliferation. For example, EGCG particularly induces cell growth inhibition by reducing HER2 and STAT3 phosphorylation in HER2 overexpressing BT474 breast cancer cells. When Her-2 positive breast cancer cells (AU565 and MCF-7) were exposed to EGCG, the inactivation of the PI3K/Akt and MAPK cascade signaling, the suppression of heregulin-b1-induced fatty acid synthase expression, and high caspase-9 activity were observed. Moreover, at even higher concentrations, EGCG enhanced the treatment sensitivity of trastuzumab-resistant HER-2 positive breast cancer cells via an increased apoptotic rate and reduced Atp production and cell growth [17].
Another polyphenol that has shown high efficacy against HER2 breast cancer cells is curcumin. It has been observed that it induced apoptosis by raising the BAX/BCL-2 ratio in the case of cells treated with 6–50 μM for 24 or 48 h. Furthermore, curcumin is much less toxic compared to classical chemotherapies. For instance, in the case of BALB-neuT transgenic mice that were administered 2 mg of curcumin in 50 μL of corn oil, three times a week for 14 or 24 weeks, a reduction in tumor proliferation and better tumor-free survival were observed, everything without potential side effects [18]. Studies linked to the second-generation curcumin analog RL66 reported excellent anti-tumorigenic potential in HER2 overexpressing SKBR3 breast cancer cells. When 1–3 μM of RL66 was used, it was observed that after 12–36 h, the intrinsic apoptosis was triggered, along with cell cycle arrest and reduced Her2 phosphorylation [17].
In addition, the Mediterranean diet, which includes various bioactive components, has been addressed in cancer treatment. Vinod et al. [19] demonstrated, on HER2 overexpressing SKBR3 breast cancer cells, that resveratrol had the ability to cancel docetaxel-associated HER2 phosphorylation, along with further activation of its related-downstream (MAPK and Akt) signaling cascades, at a concentration of 10–25 μM resveratrol and 0.1–10 nM docetaxel. The synergistic use of resveratrol and docetaxel showed enhanced cytotoxicity, which led to an increased apoptosis rate due to caspase-8,-9,-7,-3 activation and the break in their downstream target protein, PARP. Apoptotic cell death was possible due to the gathering of cells in the sub-G0 phase and DNA disruption. Additionally, the activation of Akt, ERK, Bad, JNK, BCl-2, and P38 under docetaxel activity was stopped by resveratrol pre-treatment, along with the nuclear translocation and DNA binding of Ap-1. It has also been reported that Akt offers resistance to docetaxel. The ability of resveratrol to act synergistically and as a chemosensitizing agent is linked to AKT2 downregulation by resveratrol and to the reduction in Akt-targeted anti-apoptotic protein survival and gene XIAP (which offers taxane-resistance because it promotes a premature mitotic exit [17].
Another study by Luo et al. described that EGCG, when used with paclitaxel, acts in a synergistic way by sensitizing cancer cells both in vitro and in vivo. An excellent reduction in the proliferation and an enhancement in taxol-induced apoptosis were reported in various breast cancer cell lines in vitro due to the ability of EGCG to increase the activation of c-Jun N-terminal kinases (JNKs) mediated by paclitaxel. Its sensitizing ability was also observed in vivo through the growth inhibition of 4T1 breast cancer cells in mice [20][21].
Another polyphenol that is able to potentiate epirubicin-induced apoptosis in MDA-MB-231 breast cancer cell is ferulic acid. This activity may be regulated through the Bax/Bcl-2/Caspase-3 pathway and PDI/IRE1α/PERK module of the endoplasmic reticulum stress signaling pathways. The results showed that the combined activity of ferulic acid and epirubicin enhanced the level of expression of endoplasmic reticulum stress proteins (PDI, PEPK, and IRE1α). These findings suggest that ferulic acid can act as an adjuvant in breast cancer treatment [22].
4. Ovarian Cancer
Ovarian cancer is considered as the deadliest cancer in females. The main problem associated with ovarian cancer treatment is the development of chemo-resistance. Similarly, with other cancer types, studies have focused on finding combined therapeutic strategies such as the administration of natural products. Studies have been performed on the effects of grape seed extract on the OVCAR-3, chemo-resistant cell line, and showed that it can inhibit cell growth and proliferation, while promoting the apoptotic process. The anti-proliferative activity of grape seed extract may be due to an increase in PTEN and DACT1 gene expression as well as the inhibition of the PI3K/AKT/MTOR and Wnt/β-catenin signaling pathway. Furthermore, the grape seed extract may destroy ovarian cancer cells by encouraging both extrinsic and intrinsic apoptotic pathways [23].
Additionally, resveratrol specifically acts as a multi-targeting drug by regulating signal transduction pathways that influence cell cycle progression proliferation, inflammation, metastasis, apoptosis, and angiogenesis. In the particular case of ovarian cancer, resveratrol led to a cessation of interleukin IL-6-promoted cell migration via ARH-1 activation, which is a tumor suppressor that modulates autophagy promotion [4][24].
Additionally, a number of in vitro studies have pointed out the inhibitory role of resveratrol on cellular glucose metabolism. When tested on ovarian cancer cells, resveratrol reduced the use of glucose and induced autophagy, emphasizing the conditions of nutrient deprivation [25].
Resveratrol inhibited glucose uptake, glycolysis, cell growth, invasion, and proliferation in a selective manner and promoted apoptosis without being influenced by p53 status in vitro (Figure 2). Resveratrol did not affect mRNA, GLUT1, and protein expressions, but stopped intracellular GLUT1 to reach the plasma membrane. This effect seems to be associated with the inhibitory effect of resveratrol on Akt activity. Therefore, these results highlight the fact that resveratrol can promote the apoptosis of ovarian cancer cells by affecting glucose uptake, a process that involves Akt-regulated plasma membrane GLUT1 trafficking [26].
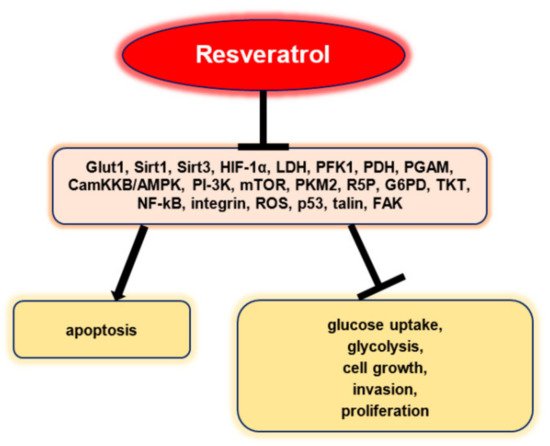
Figure 2. Resveratrol’s anti-neoplastic effects via the regulation of tumor glucose metabolism [24].
In vivo, resveratrol led to reduced glucose uptake in a mouse model, showing antineoplastic properties and the inhibition of tumor regrowth after cytostatic therapy (cisplatin) [27].
Regarding EGCG utilization, it was observed that this polyphenol enhanced the anticancer capacity of chemotherapeutic compounds in ovarian cancer. Chen et al. described that EGCG potentiated cisplatin susceptibility and reduced ovarian cancer cell growth via hydrogen peroxide (H2O2) delivery. This polyphenol potentiated the beneficial results of cisplatin up to six-fold in SKOV 3, CAOV3, and C200, a cisplatin-resistant ovarian cancer cell line. Its efficacy could be associated with the fact that it can increase intracellular H2O2 levels, meaning that higher oxidative stress could enhance the chemotherapy efficacy in ovarian cancer [20][28].
Another study by Yallapu et al. analyzed the way curcumin-based nanoparticles influence A2780CP cisplatin resistant ovarian cancer cells. Therefore, in order to enhance the curcumin pharmacokinetics in vivo, curcumin nanoparticles were conjugated with a monoclonal antibody with an affinity for tumor cells. These nanoparticles had excellent results in inhibiting the proliferation of A2780CP ovarian cancer cells, suggesting that this formulation could improve curcumin release to the tumor site and sensitize radio- and/or chemo-resistant cancer cells with high specificity [20][29].
The aflavin-3,3′-digallate (TF3) is another polyphenolic agent extracted from black tea that has shown great potential against ovarian cancer cells. As above-mentioned, this type of cancer has a low survival rate because cells usually develop cisplatin resistance. The research by Pan et al. investigated the synergy between TF3 and cisplatin in the A2780/CP70 and OVCAR3 cells. A combined pro-apoptotic effect and the arrest of cells in the G1/S phase have been observed. In addition, there was a regulation of the protein expression of cytochrome c, cleaved caspase 3/7, Bcl-2, and Bax. The synergistic use led to G1/S cell cycle arrest by the modulation of cyclin A2, D1, E1, and CDK2/4 protein expression. Additionally, the simultaneous use of these two compounds could downregulate Akt phosphorylation in both cell lines [30].
EGCG, the main component of green tea, is able to strongly bind to small molecular drugs, proteins, and DNA. Thus, it can be used for biomacromolecules and drug delivery. Chuan et al. developed a drug delivery system based on polyethylene glycol folic acid functionalized EGCG and doxorubicin for the targeted therapy of ovarian cancer. Studies have revealed that the system improved doxorubicin uptake by the SKOV3 cancer cells when compared with the system without further folic acid functionalization. Additionally, the in vitro tests showed higher toxicity and tumor growth inhibition for the folic acid functionalized system on SKOV3 cells [31].
Green tea and paclitaxel are another combination that has shown improved anti-neoplastic activity in ovarian cancer when compared with the separate effects of the two agents. The synergistic effect acts by the inhibition of Akt phosphorylation. Many studies have demonstrated that increased Akt signaling pathway activation leads to a lower apoptosis rate in multiple cancer types via phosphorylation and the inactivation of pro-apoptotic mediators such as the Bad protein [32]. The inhibition of the Akt pathway was associated with the activation of the mitochondrial apoptotic pathway characterized by an important reduction in the anti-apoptotic BCL-2 protein and a notable increase in the Bad levels, Cyt-c, cleaved-caspases-3 and -9, and Bax [33].
5. Colorectal Cancer
In 2008, the American Cancer Society stated that colorectal cancer was the third most common cancer type in Western countries and has caused around 10% of all cancer related deaths in the U.S. A concerning increase in areas that were previously at low risk such as Africa, Asia, and Latin America has also been observed. Statistics worldwide have reported differences in the incidence, showing that environmental factors play a huge role in disease development. Some of the factors that may influence colorectal cancer development are obesity, diets high in calories, and sedentariness. There are clues that indicate that the mechanism of these risk factors is controlled by hyperinsulinemia and that insulin could enhance the growth of colon tumors [34].
The antitumoral activity of resveratrol was investigated in the HCT116 and Caco 2 human colorectal cancer cell lines. The results showed that resveratrol had the ability to inhibit the proliferation of both HCT116 and Caco2 colon cancer cells and to reduce pyruvate kinase and lactate dehydrogenase glycolytic enzymes in Caco2 cells. At the same time, an enhancement in the citrate synthase activity and a reduction in glucose expenditure were reported in both cell lines. Additionally, resveratrol managed to downregulate leptin and c-Myc expression and reduce the quantity of VEGF (vascular endothelial growth factor). An activation of caspases 3 and 8 apoptotic markers and an increase in the Bax/Bcl-2 ratio was observed. The study suggested that the calorie-restriction pathway may be the cause of this activity [34].
Resveratrol loaded polyethylene glycol–polylactic acid polymeric nanoparticles were tested both in vitro and in vivo on colon cancer and they led to delayed tumor growth together with an increased survival rate. The study suggested that the antitumoral and metabolic effect of resveratrol were preserved by polymeric nanoparticle loading both in vitro and in vivo [35].
Additionally, resveratrol changes the lipidomic profile, acts on raising the oxidative capacities via the CamKKB/AMPK pathway, and reduces glycolysis, along with a reduced pentose phosphate activity and higher ATP production in the HTC116 and Caco2 colon cancer cells [36].
Resveratrol also inhibits the glycolysis and glucose uptake in HT-29 cells. This metabolic response relies on the ability of resveratrol to inhibit intracellular ROS, which further downregulates HIF-1α accumulation, glycolytic flux, and Glut-1 expression [25].
Like any other type of cell, cancer cells demand a continuous flux of nutrients to properly grow and divide. Therefore, when the blood supply requirement is not fulfilled, the cancer will eventually stop growing. Angiogenesis represents the physiological process that leads to the formation of new blood vessels from pre-existing ones. Cancers stimulate angiogenesis through the secretion of several growth factors such as VEGF, which plays the main role in angiogenesis. When cancer angiogenesis is inhibited, the malignant tissue will die. Moreover, there are some factors that play important parts in metastasis: cancer cell mobility, migration, and invasion. Hence, inhibiting at least one of these three factors will stop metastasis. EGCG showed excellent potential in reducing angiogenesis, cell mobility, migration, invasion, and metastasis markers in various human cancers. In colorectal cancer, EGCG stopped tumor growth in SW837 cells in vitro and also in vivo. This happened via activation of the VEGF/VEGFR axis through the inhibition of HIF-1a expression and some other important growth factors. These polyphenols also reduced the migration and proliferation in SW620 cells in vitro via suppression of the PAR2-AP and VIIa factor together with the ERK 1/2 and NF-jB pathways [12].
Honey polyphenols are also great antioxidants and have powerful anticancer properties. Cinaciosi et al. studied the activity of Manuka honey on cancer stem cells such as from colorectal cancer (HCT116 cell line) enriched by the in vitro sphere-forming assay. It has been observed that Manuka honey decreased the volume of the entire culture spheroids, modifying their morphological parameters and promoted the apoptosis and intracellular ROS increase in these cells. Moreover, it reduced the mRNA expression ABCG2—an ABC transporter and influenced the self-renewal ability via downregulation of the mRNA expression of one of the receptor membranes of the Wnt/β-catenin pathway [37].
Resveratrol was studied in vitro on AK4-knockdown colon cancer cells (SW480 and SW620) and showed that it could diminish the invasion and metastasis of colon cancer cells by reversing the expression of EMT (epithelial–mesenchymal transition) markers via the AKT/GSK-3β/Snail pathway. Actually, AKT1 can act as an important regulator of EMT colon cancer cells and be a possible therapeutic target for colon cancer [38][39].
Djulis is a cereal that contains many polyphenols and fibers that have been reported to prevent colon malignancies. Lee et al. studied its effects on rats and discovered that the polyphenol content could reduce oxidative stress and modulate proteins involved in anti-apoptosis, pro-apoptosis, and proliferation to avoid colorectal cancer progression. Hence, in the future, djulis may be a great colorectal cancer chemopreventive product. Djulis can inhibit the generation of colonic preneoplastic lesions (ACF and MDF) in DMH-induced colon carcinogenesis in rats. In the colon of rats, djulis simultaneously raised the expression of proapoptosis-related proteins (Bax and caspase-9) and the activity of antioxidant enzymes (CAT and SOD). Additionally, p53, a protein related to proliferation (PCNA) and a protein connected to the prevention of apoptosis were all suppressed by Djulis (Bcl-2) (Figure 3) [39][40].
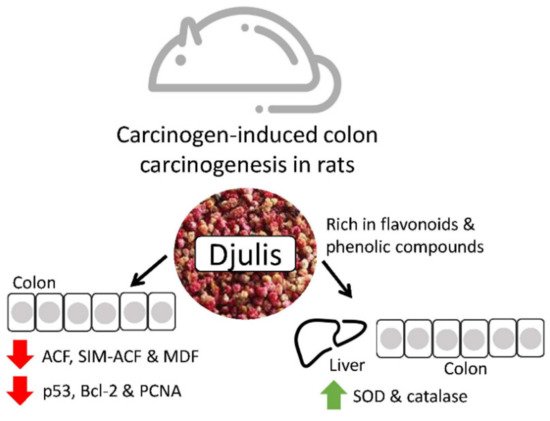
Figure 3. The effects of djulis in colon and liver cancer [40].
6. Osteosarcoma
Osteosarcoma is the most common primary malignant bone tumor in children and adolescents. The current treatment of osteosarcoma consists of surgical resection, radiotherapy, and chemotherapy. There are several existing drugs (salinomycin, cisplatin, doxorubicin, methotrexate, 5-fluorouracil, oxaliplatin, etc.) that are used for osteosarcoma treatment, but they have strong adverse effects and are effective when used in the initial stages. Therefore, more natural compounds with less side effects and lower toxicity on normal cells could be a great alternative, especially if synergies can be developed. One of these compounds is curcumin, which has been investigated for its pleiotropic effects, antioxidant, anti-inflammatory, antibacterial, and pro-wound healing activity, and for its ability to form curcumin–metal complexes [41].
Curcumin, a well-known polyphenol that has great antitumoral properties, was loaded in biodegradable copolymer coatings (polyvinyl alcohol-polyethylene glycol) through the MAPLE technique (matrix assisted pulsed laser evaporation) and tested on MG-63 cells, suggesting an improved reduction in osteosarcoma cell viability and proliferation [41].
Oxidative stress has various negative effects including osteoblast cell differentiation via the reduction of alkaline phosphatase RUNX2 differentiation markers and colony forming unit formation. Thus, antioxidant compounds such as polyphenols could act in a positive manner by protecting bone metabolism through the stimulation of osteoblast differentiation and the limitation of bone resorption. These protective properties have also been accomplished by other polyphenols such as EGCG and genistein. The loading of polyphenols and the optimization of delivery methods to the host tissue are important elements that influence the compound efficacy [42].
The normal process of bone reconstruction is characterized by continuous and alternate formation and resorption processes that are influenced by several molecular signaling pathways. However, the uneven effects of factors in these pathways caused reduced osteoblast activity and enhanced osteoclast activity. This means a disruption in bone formation and resorption, which leads to deficient bone regeneration. Many studies have demonstrated that polyphenols are important in the modulation of bone regeneration, primarily due to their antioxidant properties, which reduces the inflammatory response and stimulates the normal process of bone regeneration. Moon et al. reported that curcumin at a concentration of 5 μM is able to successfully clean free radicals and downregulate NFκB expression (the most important transduction factor involved in inflammation). Polyphenols can also influence the activation and modulation of antioxidant enzymes such as superoxide dismutase, catalase, and glutathione peroxidase, the main protector against ROS. The direct antioxidant activity, together with the stimulation of antioxidant enzymes, could successfully clean the existing ROS and prevent mitochondria from generating more free radicals. This helps with inhibiting osteoblast apoptosis via the suppression of the p53 apoptotic signal [5].
Nani et al. investigated the pro-apoptotic properties of Pennisetum glaucum, a pearl millet phenolic compound (PGPC), on U-2OS osteosarcoma cells. PGPC led to U-2OS cell death, proportional to the dose. PGPC downregulates AKT downstream and upstream effectors that are related to SAPK/JNK and p38 upregulation and high [Ca2+], leading to cell cycle arrest and caspase-dependent apoptosis in U-2OS osteosarcoma cells [43].
It has been demonstrated that resveratrol reduces the cell viability, self-renewal capacity, and tumorigenesis of osteosarcoma cells, while not causing any harm to normal osteoblast cells. Resveratrol also reduced the cytokine synthesis and blocked the JAK2/STAT3 signaling pathway, which influenced the reduction in the cancer stem cell marker, CD133. The obtained data showed that resveratrol stopped osteosarcoma cell proliferation and tumorigenesis capacity, which was linked to the cytokine inhibition related JAK2/STAT3 signaling blockage [44].
MicroRNAs represent a category of short noncoding RNAs and are strongly involved in gene regulation, pathogenesis, and human cancer progression. Zhu et al. studied EGCG activity against osteosarcoma. The analysis of cellular function reported that EGCG could reduce cell proliferation, promote cell cycle arrest, and induce osteosarcoma cell apoptosis in vitro, while also stop transplanted tumor growth in vivo. A series of analyses such as RT-qPCR and miRNA microarrays were performed and revealed that miR-1 was strongly upregulated in U-2OS and MG-63 cells treated with EGCG in a direct correlation with time and dose. The miR-1 downregulation by the inhibitor resembles the attenuated EGCG-induced inhibition on osteosarcoma cell growth. It was established that miR-1 was often reduced in clinical osteosarcoma tissues. Additionally, EGCG and miR-1 mimicked the inhibited c-MET expression, and mixt treatment with EGCG and c-MET inhibitor (crizotinib) improved the inhibitory effect on U-2OS and MG-63 growth. These results show that EGCG could have anticancer activity on osteosarcoma cells via the regulation of miR-1/c-MET interaction [45].
In the case of osteosarcoma, various studies have demonstrated the benefits of curcumin, which may stimulate U-2OS, MG-63, and HOS cells apoptosis based on several signaling pathways. Moreover, curcumin has also been reported to inhibit proliferation, invasion, and metastasis in osteosarcoma. Therefore, curcumin has many great properties that play important roles in osteosarcoma treatment. Naboneeta and Susmita reported that an implant based on curcumin loaded hydroxyapatite-coated titanium improved MG-63 cytotoxicity in vitro [46]. Another study by his group suggested that curcumin incorporated in a 3D-printed calcium phosphate scaffold showed selective toxicity toward MG-63 cells and supported the normal proliferation of osteoblasts [47]. Another asset of this combination approach is the increased accumulation of curcumin in the damaged tissue area. Because of some curcumin disadvantages such as extensive first pass metabolism and poor bioavailability [48], conventional delivery strategies cannot overcome these problems. By incorporating curcumin in such materials, it can properly accumulate in the target area, resulting in a pharmacological potency boost [49].
Table 2 contains the in vitro/in vivo effects of various polyphenols against different cancer types.
| Polyphenol | In Vitro/In Vivo Study | Cancer Type | Dose | Effect | Ref. |
|---|---|---|---|---|---|
| Curcumin | In vitro (melanoma cell culture) | Skin cancer | 25 µM | Melanoma cell death associated with mPTP opening | [52] |
| EGCG | In vitro (MCF-7, MDA-MB-231and T47D cells) | Breast cancer | 1–40 μM | Inhibiting estrogen-induced cancer cell proliferation, down-regulating ERα, inhibiting metastasis | [53][54] |
| Apigenin | In vivo (BALB/c-nude mice) | Breast cancer | 5–25 mg/kg | Inducing cell cycle arrest through epigenetic change | [55] |
| Quercetin | In vivo (BALB/c nude mice) | Breast cancer | 34 mg/kg | Inhibiting angiogenesis | [56] |
| Genistein | In vitro (HeLa cells) | Cervical cancer | 100 μM | Inducing apoptosis, cell cycle arrest, suppressing cell migration | [57] |
| Resveratrol | In vitro (PC3 and DU145 cells) | Prostate cancer | 25–100 μM | Inducing autophagy-mediated cell death | [58] |
| Gallic acid | In vitro (HepG2 and SMMC-7721 cells) |
Liver cancer | 22.1–28.5 μg/mL | Inducing apoptosis | [59] |
| EGCG | In vitro (HT-29 cells) | Colorectal cancer | 1–50 μM | Inducing epigenetic alteration, apoptosis, MAPK and Akt pathways activation | [60] |
| Resveratrol | In vivo (genetically engineered mouse model for sporadic colorectal cancer) | Colorectal cancer | equal to 105 and 210 mg for human | Suppressing tumor development by modulation of Kras | [61] |
This entry is adapted from the peer-reviewed paper 10.3390/ijms231810244
References
- Lewandowska, H.; Kalinowska, M.; Lewandowski, W.; Stępkowski, T.M.; Brzóska, K. The role of natural polyphenols in cell signaling and cytoprotection against cancer development. J. Nutr. Biochem. 2016, 32, 1479–1490.
- Oyenihi, A.B.; Smith, C. Are polyphenol antioxidants at the root of medicinal plant anti-cancer success? J. Ethnopharmacol. 2019, 229, 54–72.
- Khan, H.; Reale, M.; Ullah, H.; Sureda, A.; Tejada, S.; Wang, Y.; Zhang, Z.-J.; Xiao, J. Anti-cancer effects of polyphenols via targeting p53 signaling pathway: Updates and future directions. Biotechnol. Adv. 2020, 38, 107385.
- Patra, S.; Mishra, S.R.; Behera, B.P.; Mahapatra, K.K.; Panigrahi, D.P.; Bhol, C.S.; Praharaj, P.P.; Sethi, G.; Patra, S.K.; Bhutia, S.K. Autophagy-modulating phytochemicals in cancer therapeutics: Current evidences and future perspectives. Semin. Cancer Biol. 2020, 80, 205–217.
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74.
- Di Domenico, F.; Foppoli, C.; Coccia, R.; Perluigi, M. Antioxidants in cervical cancer: Chemopreventive and chemotherapeutic effects of polyphenols. Biochim. Biophys. Acta 2011, 1822, 737–747.
- Sajadimajd, S.; Bahramsoltani, R.; Iranpanah, A.; Kumar Patra, J.; Das, G.; Gouda, S.; Rahimi, R.; Rezaeiamiri, E.; Cao, H.; Giampieri, F.; et al. Advances on Natural Polyphenols as Anticancer Agents for Skin Cancer. Pharmacol. Res. 2020, 151, 104584.
- Niedzwiecki, A.; Roomi, M.W.; Kalinovsky, T.; Rath, M. Anticancer Efficacy of Polyphenols and Their Combinations. Nutrients 2016, 8, 552.
- Singh, T.; Katiyar, S.K. Green tea polyphenol, (−)-epigallocatechin-3-gallate, induces toxicity in human skin cancer cells by targeting β-catenin signaling. Toxicol. Appl. Pharmacol. 2013, 273, 418–424.
- Kumar, Y.; Bhatia, A. Chapter 49—Polyphenols and Skin Cancers. In Polyphenols in Human Health and Disease; Watson, R.R., Preedy, V.R., Zibadi, S., Eds.; Academic Press: San Diego, CA, USA, 2014; pp. 643–653.
- Philips, N.; Siomyk, H.; Bynum, D.; Gonzalez, S. Chapter 26-Skin Cancer, Polyphenols, and Oxidative Stress. In Cancer; Preedy, V., Ed.; Academic Press: San Diego, CA, USA, 2014; pp. 265–270.
- Rady, I.; Mohamed, H.; Rady, M.; Siddiqui, I.A.; Mukhtar, H. Cancer preventive and therapeutic effects of EGCG, the major polyphenol in green tea. Egypt J. Basic Appl. Sci. 2018, 5, 1–23.
- Kalekhan, F.; Bala, N.; Rao, S.; Pais, M.L.J.; Adnan, M.; Sajan, S.; Baliga, M.S. 9—Usefulness of grape seed polyphenols in the prevention of skin cancer: A mini review. In Functional Foods in Cancer Prevention and Therapy; Kabir, Y., Ed.; Academic Press: San Diego, CA, USA, 2020; pp. 159–167.
- Chen, X.J.; Chang, L.L.; Qu, Y.; Liang, J.N.; Jin, W.S.; Xia, X.J. Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the down-regulation of TLR4. Int. J. Immunopath. Ph. 2018, 31, 394632017739531.
- Poschner, S.; Maier-Salamon, A.; Thalhammer, T.; Jäger, W. Resveratrol and other dietary polyphenols are inhibitors of estrogen metabolism in human breast cancer cells. J. Steroid Biochem. Mol. Biol. 2019, 190, 11–18.
- Das, N.; Nagpal, N.; Bankura, S.S.; Kumar, K. Breast Cancer and Phytochemicals: The Current Perspective. IIOAB J. 2019, 10, 27–33.
- Zabaleta, M.E.; Forbes-Hernández, T.Y.; Simal-Gandara, J.; Quiles, J.L.; Cianciosi, D.; Bullon, B.; Giampieri, F.; Battino, M. Effect of polyphenols on HER2-positive breast cancer and related miRNAs: Epigenomic regulation. Food Res. Int. 2020, 137, 109623.
- Masuelli, L.; Benvenuto, M.; Fantini, M.; Marzocchella, L.; Sacchetti, P.; Di Stefano, E.; Tresoldi, I.; Izzi, V.; Bernardini, R.; Palumbo, C.; et al. Curcumin induces apoptosis in breast cancer cell lines and delays the growth of mammary tumors in neu transgenic mice. J. Biol. Regul. Homeost. Agents 2013, 27, 105–119.
- Vinod, B.S.; Nair, H.H.; Vijayakurup, V.; Shabna, A.; Shah, S.; Krishna, A.; Pillai, K.S.; Thankachan, S.; Anto, R.J. Resveratrol chemosensitizes HER-2-overexpressing breast cancer cells to docetaxel chemoresistance by inhibiting docetaxel-mediated activation of HER-2–Akt axis. Cell Death Discov. 2015, 1, 15061.
- Fantini, M.; Benvenuto, M.; Masuelli, L.; Frajese, G.V.; Tresoldi, I.; Modesti, A.; Bei, R. In vitro and in vivo antitumoral effects of combinations of polyphenols, or polyphenols and anticancer drugs: Perspectives on cancer treatment. Int. J. Mol. Sci. 2015, 16, 9236–9282.
- Luo, T.; Wang, J.; Yin, Y.; Hua, H.; Jing, J.; Sun, X.; Li, M.; Zhang, Y.; Jiang, Y. (-)-Epigallocatechin gallate sensitizes breast cancer cells to paclitaxel in a murine model of breast carcinoma. Breast Cancer Res. BCR 2010, 12, R8.
- Cheng, W.J.; Zhang, P.P.; Luo, Q.Q.; Deng, S.M.; Jia, A.Q. The chemosensitizer ferulic acid enhances epirubicin-induced apoptosis in MDA-MB-231 cells. J. Funct. Foods 2020, 73, 104130.
- Homayoun, M.; Targhi, R.; Soleimani, M. Anti-proliferative and anti-apoptotic effects of grape seed extract on chemo-resistant OVCAR-3 ovarian cancer cells. Res. Pharm. Sci. 2020, 15, 390.
- Brockmueller, A.; Sameri, S.; Liskova, A.; Zhai, K.V.; Varghese, E.; Samuel, S.M.; Busselberg, D.; Kubatka, P.; Shakibaei, M. Resveratrol’s Anti-Cancer Effects through the Modulation of Tumor Glucose Metabolism. Cancers 2021, 13, 188.
- Jung, K.-H.; Lee, J.H.; Thien Quach, C.H.; Paik, J.-Y.; Oh, H.; Park, J.W.; Lee, E.J.; Moon, S.-H.; Lee, K.-H. Resveratrol Suppresses Cancer Cell Glucose Uptake by Targeting Reactive Oxygen Species–Mediated Hypoxia-Inducible Factor-1α Activation. J. Nucl. Med. 2013, 54, 2161–2167.
- Gwak, H.; Haegeman, G.; Tsang, B.K.; Song, Y.S. Cancer-specific interruption of glucose metabolism by resveratrol is mediated through inhibition of Akt/GLUT1 axis in ovarian cancer cells. Mol. Carcinog. 2015, 54, 1529–1540.
- Yang, S.H.; Kim, J.S.; Oh, T.J.; Kim, M.S.; Lee, S.W.; Woo, S.K.; Cho, H.S.; Choi, Y.H.; Kim, Y.H.; Rha, S.Y.; et al. Genome-scale analysis of resveratrol-induced gene expression profile in human ovarian cancer cells using a cDNA microarray. Int. J. Oncol. 2003, 22, 741–750.
- Chan, M.M.; Soprano, K.J.; Weinstein, K.; Fong, D. Epigallocatechin-3-gallate delivers hydrogen peroxide to induce death of ovarian cancer cells and enhances their cisplatin susceptibility. J. Cell. Physiol. 2006, 207, 389–396.
- Yallapu, M.M.; Maher, D.M.; Sundram, V.; Bell, M.C.; Jaggi, M.; Chauhan, S.C. Curcumin induces chemo/radio-sensitization in ovarian cancer cells and curcumin nanoparticles inhibit ovarian cancer cell growth. J. Ovarian Res. 2010, 3, 11.
- Pan, H.; Li, J.; Rankin, G.O.; Rojanasakul, Y.; Tu, Y.; Chen, Y.C. Synergistic effect of black tea polyphenol, theaflavin-3,33,3n cancer cells and curcumin nan cisplatin resistant human ovarian cancer cells. J. Funct. Foods 2018, 46, 1–11.
- Chuan, D.; Mu, M.; Hou, H.; Zhao, N.; Li, J.; Tong, A.; Zou, B.; Chen, H.; Han, B.; Guo, G. Folic acid-functionalized tea polyphenol as a tumor-targeting nano-drug delivery system. Mater. Des. 2021, 206, 109805.
- Rana, C.; Piplani, H.; Vaish, V.; Nehru, B.; Sanyal, S.N. Downregulation of PI3-K/Akt/PTEN pathway and activation of mitochondrial intrinsic apoptosis by Diclofenac and Curcumin in colon cancer. Mol. Cell. Biochem. 2015, 402, 225–241.
- Panji, M.; Behmard, V.; Zare, Z.; Malekpour, M.; Nejadbiglari, H.; Yavari, S.; Nayerpour Dizaj, T.; Safaeian, A.; Bakhshi, A.; Abazari, O.; et al. Synergistic effects of green tea extract and paclitaxel in the induction of mitochondrial apoptosis in ovarian cancer cell lines. Gene 2021, 787, 145638.
- Fouad, M.A.; Agha, A.M.; Merzabani, M.M.A.; Shouman, S.A. Resveratrol inhibits proliferation, angiogenesis and induces apoptosis in colon cancer cells: Calorie restriction is the force to the cytotoxicity. Hum. Exp. Toxicol. 2013, 32, 1067–1080.
- Jung, K.-H.; Lee, J.H.; Park, J.W.; Quach, C.H.T.; Moon, S.-H.; Cho, Y.S.; Lee, K.-H. Resveratrol-loaded polymeric nanoparticles suppress glucose metabolism and tumor growth in vitro and in vivo. Int. J. Pharm. 2015, 478, 251–257.
- Saunier, E.; Antonio, S.; Regazzetti, A.; Auzeil, N.; Laprévote, O.; Shay, J.W.; Coumoul, X.; Barouki, R.; Benelli, C.; Huc, L.; et al. Resveratrol reverses the Warburg effect by targeting the pyruvate dehydrogenase complex in colon cancer cells. Sci. Rep. 2017, 7, 6945.
- Cianciosi, D.; Forbes-Hernández, T.Y.; Ansary, J.; Gil, E.; Amici, A.; Bompadre, S.; Simal-Gandara, J.; Giampieri, F.; Battino, M. Phenolic compounds from Mediterranean foods as nutraceutical tools for the prevention of cancer: The effect of honey polyphenols on colorectal cancer stem-like cells from spheroids. Food Chem. 2020, 325, 126881.
- Yuan, L.; Zhou, M.; Huang, D.; Wasan, H.S.; Zhang, K.; Sun, L.; Huang, H.; Ma, S.; Shen, M.; Ruan, S. Resveratrol inhibits the invasion and metastasis of colon cancer through reversal of epithelial-mesenchymal transition via the AKT/GSK-3β/Snail signaling pathway. Mol. Med. Rep. 2019, 20, 2783–2795.
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9.
- Lee, C.-W.; Chen, H.-J.; Xie, G.-R.; Shih, C.-K. Djulis (Chenopodium Formosanum) Prevents Colon Carcinogenesis via Regulating Antioxidative and Apoptotic Pathways in Rats. Nutrients 2019, 11, 2168.
- Tirca, I.; Mitran, V.; Marascu, V.; Brajnicov, S.; Ion, V.; Stokker-Cheregi, F.; Popovici, I.A.; Cimpean, A.; Dinca, V.; Dinescu, M. In vitro testing of curcumin based composites coatings as antitumoral systems against osteosarcoma cells. Appl. Surf. Sci. 2017, 425, 1040–1051.
- Shavandi, A.; Bekhit, A.E.-D.A.; Saeedi, P.; Izadifar, Z.; Bekhit, A.A.; Khademhosseini, A. Polyphenol uses in biomaterials engineering. Biomaterials 2018, 167, 91–106.
- Nani, A.; Belarbi, M.; Murtaza, B.; Benammar, C.; Merghoub, T.; Rialland, M.; Akhtar Khan, N.; Hichami, A. Polyphenols from Pennisetum glaucum grains induce MAP kinase phosphorylation and cell cycle arrest in human osteosarcoma cells. J. Funct. Foods 2019, 54, 422–432.
- Peng, L.; Jiang, D. Resveratrol eliminates cancer stem cells of osteosarcoma by STAT3 pathway inhibition. PLoS ONE 2018, 13, e0205918.
- Zhu, K.; Wang, W. Green tea polyphenol EGCG suppresses osteosarcoma cell growth through upregulating miR-1. Tumour Biol. J. Int. Soc. Oncodevelopmental Biol. Med. 2016, 37, 4373–4382.
- Sarkar, N.; Bose, S. Controlled Delivery of Curcumin and Vitamin K2 from Hydroxyapatite-Coated Titanium Implant for Enhanced in Vitro Chemoprevention, Osteogenesis, and in Vivo Osseointegration. ACS Appl. Mater. Interfaces 2020, 12, 13644–13656.
- Sarkar, N.; Bose, S. Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering. ACS Appl. Mater. Interfaces 2019, 11, 17184–17192.
- Adiwidjaja, J.; McLachlan, A.J.; Boddy, A.V. Curcumin as a clinically-promising anti-cancer agent: Pharmacokinetics and drug interactions. Expert Opin. Drug Metab. Toxicol. 2017, 13, 953–972.
- Xu, C.; Wang, M.; Guo, W.; Sun, W.; Liu, Y. Curcumin in Osteosarcoma Therapy: Combining With Immunotherapy, Chemotherapeutics, Bone Tissue Engineering Materials and Potential Synergism With Photodynamic Therapy. Front. Oncol. 2021, 11, 672490.
- Zhou, Y.; Zheng, J.; Li, Y.; Xu, D.P.; Li, S.; Chen, Y.M.; Li, H.B. Natural Polyphenols for Prevention and Treatment of Cancer. Nutrients 2016, 8, 515.
- Panahi, Y.; Fazlolahzadeh, O.; Atkin, S.L.; Majeed, M.; Butler, A.E.; Johnston, T.P.; Sahebkar, A. Evidence of curcumin and curcumin analogue effects in skin diseases: A narrative review. J. Cell. Physiol. 2019, 234, 1165–1178.
- Qiu, Y.; Yu, T.; Wang, W.; Pan, K.; Shi, D.; Sun, H. Curcumin-induced melanoma cell death is associated with mitochondrial permeability transition pore (mPTP) opening. Biochem. Biophys. Res. Commun. 2014, 448, 15–21.
- Deb, G.; Thakur, V.S.; Limaye, A.M.; Gupta, S. Epigenetic induction of tissue inhibitor of matrix metalloproteinase-3 by green tea polyphenols in breast cancer cells. Mol. Carcinog. 2015, 54, 485–499.
- De Amicis, F.; Russo, A.; Avena, P.; Santoro, M.; Vivacqua, A.; Bonofiglio, D.; Mauro, L.; Aquila, S.; Tramontano, D.; Fuqua, S.A.; et al. In vitro mechanism for downregulation of ER-α expression by epigallocatechin gallate in ER+/PR+ human breast cancer cells. Mol. Nutr. Food Res. 2013, 57, 840–853.
- Tseng, T.H.; Chien, M.H.; Lin, W.L.; Wen, Y.C.; Chow, J.M.; Chen, C.K.; Kuo, T.C.; Lee, W.J. Inhibition of MDA-MB-231 breast cancer cell proliferation and tumor growth by apigenin through induction of G2/M arrest and histone H3 acetylation-mediated p21(WAF1/CIP1) expression. Environ. Toxicol. 2017, 32, 434–444.
- Zhao, X.; Wang, Q.; Yang, S.; Chen, C.; Li, X.; Liu, J.; Zou, Z.; Cai, D. Quercetin inhibits angiogenesis by targeting calcineurin in the xenograft model of human breast cancer. Eur. J. Pharmacol. 2016, 781, 60–68.
- Hussain, A.; Harish, G.; Prabhu, S.A.; Mohsin, J.; Khan, M.A.; Rizvi, T.A.; Sharma, C. Inhibitory effect of genistein on the invasive potential of human cervical cancer cells via modulation of matrix metalloproteinase-9 and tissue inhibitors of matrix metalloproteinase-1 expression. Cancer Epidemiol. 2012, 36, e387–e393.
- Selvaraj, S.; Sun, Y.; Sukumaran, P.; Singh, B.B. Resveratrol activates autophagic cell death in prostate cancer cells via downregulation of STIM1 and the mTOR pathway. Mol. Carcinog. 2016, 55, 818–831.
- Sun, G.; Zhang, S.; Xie, Y.; Zhang, Z.; Zhao, W. Gallic acid as a selective anticancer agent that induces apoptosis in SMMC-7721 human hepatocellular carcinoma cells. Oncol. Lett. 2016, 11, 150–158.
- Cerezo-Guisado, M.I.; Zur, R.; Lorenzo, M.J.; Risco, A.; Martín-Serrano, M.A.; Alvarez-Barrientos, A.; Cuenda, A.; Centeno, F. Implication of Akt, ERK1/2 and alternative p38MAPK signalling pathways in human colon cancer cell apoptosis induced by green tea EGCG. Food Chem. Toxicol. Int. J. Publ. Br. Ind. Biol. Res. Assoc. 2015, 84, 125–132.
- Saud, S.M.; Li, W.; Morris, N.L.; Matter, M.S.; Colburn, N.H.; Kim, Y.S.; Young, M.R. Resveratrol prevents tumorigenesis in mouse model of Kras activated sporadic colorectal cancer by suppressing oncogenic Kras expression. Carcinogenesis 2014, 35, 2778–2786.
This entry is offline, you can click here to edit this entry!
