Living species are continuously subjected to all extrinsic forms of reactive oxidants and others that are produced endogenously. There is extensive literature on the generation and effects of reactive oxygen species (ROS) in biological processes, both in terms of alteration and their role in cellular signaling and regulatory pathways. Cells produce ROS as a controlled physiological process, but increasing ROS becomes pathological and leads to oxidative stress and disease. The induction of oxidative stress is an imbalance between the production of radical species and the antioxidant defense systems, which can cause damage to cellular biomolecules, including lipids, proteins and DNA. Cellular and biochemical experiments have been complemented in various ways to explain the biological chemistry of ROS oxidants. However, it is often unclear how this translates into chemical reactions involving redox changes.
Note: The following contents are extract from your paper. The entry will be online only after author check and submit it.
1. Introduction
In chemistry, a free radical (FR) is a relatively stable species that contains one or more unpaired electrons and can react with other molecules, either by donating its unpaired electron to another molecule or by taking it away from another molecule to increase stability. In this way it converts the molecule with which it reacts into another FR, so a common feature of FR reactions is the chain process: one radical gives rise to another radical. This process only ceases when two FRs react with each other.
In a biological context, reactive oxygen species (ROS) are formed as a natural by-product of cellular aerobic metabolism. Mitochondrial respiration is a significant cause of reactive oxygen species (ROS) [
1]. In addition to mitochondria, ROS are produced by a variety of enzymes such as NADPH oxidases (NOXs), xanthine oxidase, nitric oxide synthase, and peroxisomal constituents [
2]. They are also produced by ionizing and UV radiation, as well as by the metabolism of a wide range of drugs and xenobiotics. In the endoplasmic reticulum, oxidants are released during the folding of proteins and the formation of disulphide bonds. They are highly reactive chemical molecules derived from the ability of the O
2 molecule to accept electrons [
3], generating subsequent unstable molecules such as superoxide anion (
•O
2−), hydrogen peroxide (H
2O
2), hydroxyl radical (OH
−), and singlet oxygen (
1O
2−), produced by all kinds of cells. Stationary (physiological) levels of ROS are intrinsic to the normal functioning of cells, fulfilling the functions of cell signalling and homeostasis [
4]. On the other hand, when they are produced in excess or when cellular defences are not able to metabolise them, oxidative stress (OS) damage occurs [
5].
2. Genesis and Effects of ROS
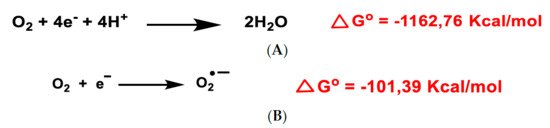
All aerobic organisms need oxygen O2 for efficient energy production. Molecular O2 contains two unpaired electrons, but it is weakly reactive because they are located in different molecular orbitals and have a parallel spin. Consequently, oxygen preferentially accepts electrons one at a time. The ultimate goal of the electron transport chain ETC in the inner mitochondrial membrane (complexes I to V) is the reduction of the oxygen molecule to produce water. The first step of the O2 reduction reaction occurs spontaneously (A).
Figure 1. Gibbs free energy of O2 reduction yielding water (A) and the anion superoxide (B). Gibbs free energy calculated with Gaussian 09 software, revision D.01, method B3LYP/6-31G(d). Authors cited Note 1, after References.
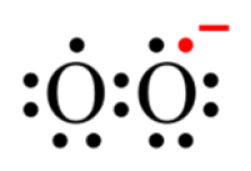
Univalent reduction of O2 gives rise to the anion superoxide (B), which results from the addition of an electron filling one of the two uncompleted molecular orbitals, leaving a charged ionic species with a single unpaired electron and a net negative charge of −1 ().
Figure 2. Lewis structure of the anion superoxide.
Superoxide
•O
2− is detrimental and is mainly produced as a by-product of mitochondrial respiration (especially in Complexes I and III, in the electron transport chain ETC) [
6], where a small percentage of the electrons in the ETC chain escape from it, as well as by several other enzymes, which catalyse the electron transfer directly to molecular oxygen under strongly reducing conditions, as occurs in the mitochondrial matrix. It is also generated in the immune system to eliminate invading micro-organisms. In phagocytes, the enzyme NADPH oxidase produces
•O
2− in large quantities for use in the oxygen-dependent destruction mechanisms of invading pathogens [
7].
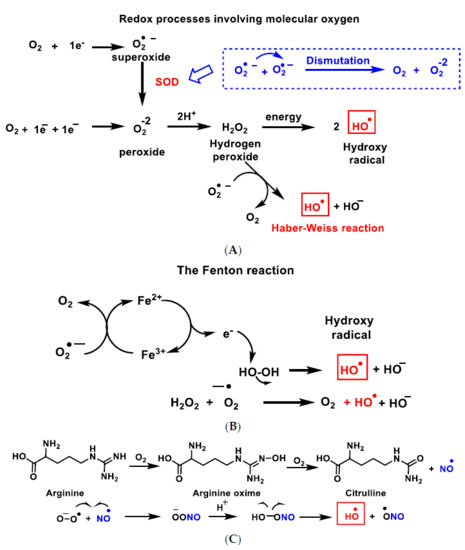
The hydroxyl radical
•OH (A–C), the most powerful ROS oxidant, is formed during the Haber–Weiss reaction [
8], by the Fenton reaction [
9] or by decomposition of peroxynitrite [
10], and has a very short half-life (10
−9 s) and high reactivity.
Figure 3. Reactive species ROS and RNS formed in the mitochondrial matrix by the Haber–Weiss reaction (A), the Fenton reaction (B) or by decomposition of peroxynitrite (C).
O
2 and ROS reduction are reactions in which the final product is water and have a negative Gibbs free energy, ∆G
o ≤ 0, so they occur spontaneously [
11]. A small percentage of the electrons in the ETC transport chain, 0.1 to 2% of cases, pass through the chain and reach the mitochondrial matrix, where they prematurely reduce molecular oxygen O
2 to superoxide ion
•O
2−, the precursor of most reactive oxygen species.
3. Dual Role of ROS: Physiological and Pathological
ROS can be induced by exogenous sources such as tobacco, pollution, smoke, drugs, xenobiotics or ionising radiation, leading to irreversible effects on tissue development in animals and plants. In these, abiotic factors such as lack of water or high temperature can influence their emission. ROS play an important role in physio- and pathological processes. Mitochondria are particularly susceptible to oxidative damage, as electrons escaping from the ETC electron transport chain in the inner membrane react with oxygen to produce a superoxide anion. This anion is unstable and cannot cross membranes, but it is rapidly converted to hydrogen peroxide, which is permeable to the membrane. It can then undergo the Fenton reaction to produce the hydroxyl radical, which is highly reactive in the mitochondrial matrix. Elevated levels of ROS lead to increased mitochondrial DNA (mtDNA) damage.
In eukaryotic cells, ROS are mainly produced by biochemical reactions in mitochondrial cellular respiration processes (complex I and III, located in inner membrane). Transmembrane NADPH oxidases (NOXs) [
12] and the mitochondrial electron transport chain (ETC) are the major endogenous enzymatic sources of
•O
2− and H
2O
2. However, they also come from sources such as peroxisomes, xanthine oxidase (XO), lipo- and cyclo-oxygenase and cytochrome P450 in endoplasmic reticulum [
3]. In mitochondria, physiological levels of
•O
2− and H
2O
2 participate in redox signalling, but their production is significantly enhanced during oxidative stress conditions, producing an imbalance between ROS production and the action of the antioxidant defence system. They include SOD enzymes that reduce O
2 into H
2O
2 (Zn-Cu SOD or SOD1 in cytosol and intermembrane space in mitochondria and Mn-SOD [
13] or SOD2 in matrix mitochondria), catalase, glutathione peroxidases and thioredoxin reductase that convert levels of H
2O
2 into H
2O and O
2.
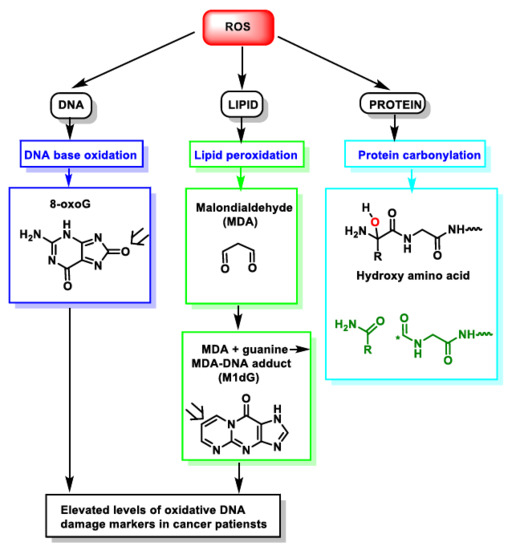
During episodes of environmental stress, oxidative damage (OS) in cells can increase dramatically, causing damage to their structures [
14]. Generally, the harmful effects of ROS in the cell include (i) damage on DNA or RNA [
15]; (ii) lipid peroxidation of polyunsaturated fatty acids (such as membrane phospholipids) [
16]; and (iii) oxidation of proteins [
17], . They cause irreversible damage to DNA, lipids and enzymes present in the cell cytosol, as they oxidise and modify cellular components and prevent them from carrying out their original functions.
Figure 4. ROS action on DNA, lipids and proteins lead to DNA base oxidation, lipid peroxidation and protein carbonylation, respectively. * Unpaired electron.
This entry is adapted from the peer-reviewed paper 10.3390/ijms22094642