1. Introduction
Aging is arguably one of the most complex molecular biological processes. The majority of eukaryotic organisms undergo the aging process as progressive levels of cellular and tissue damage accumulate across the organism’s lifetime. While bewilderingly complex, aging can be deconstructed as a molecular biological process to reveal a core series of functions that represent a consistent signature that lends itself to potential generic therapeutic interventions. To this end, considerable research has suggested that through the targeting of these key signature features a tractable ability to control the aging process may be engineered. Here we discuss how such a novel target may have been recently identified.
1.1. Aging and Aging-Related Disorders
The increase in the world’s elderly population has caused an increased prevalence of aging-related chronic disease conditions, such as neurodegenerative disorders (e.g., Alzheimer’s disease (AD)), cardiovascular disease, arthritis, chronic kidney disease, and Type II Diabetes Mellitus (T2DM) [
1]. Aging is a degradative neurometabolic process affecting every organ, and it drives the progression of a multitude of diseases. Aging is a complex multi-factorial process and, while some contributing factors may be unique to each individual, there are many common etiological factors across populations [
1,
2]. Aging is typified by the accumulation of molecular damage, causing progressive loss of an organism’s optimal function, eventually leading to systemic dysfunction and death [
1,
3].
Aging and many aging-related disorders involve perturbed energy balance [
3]. Regulation of glucose metabolism, via the canonical insulinotropic system, has been shown to be a crucial regulator of the rate of aging [
4]. The alteration of energy-controlling organelles and a significant reduction in glucose uptake are a sign of metabolic dysfunction. In times of stress or temporary depletion of glucose supplies, cellular energy metabolism will reflexively shift from glucose to adipose or protein metabolism to guarantee energy production. This metabolic change can cause oxidative stress [
5], as the catabolism of these alternative energy sources is less energy efficient and yields lower ATP. The Harman free radical/oxidative stress theory stipulates that physiological iron and other metals in the body cause reactive oxygen species (ROS) accumulation in cells, as a by-product of normal redox reactions. ROS are essentially by-products of a variety of pathways that are involved in aerobic metabolism. The accumulation of oxidative stress constitutes one of the most realistic hypotheses of aging and neurodegenerative disorders [
1]. This oxidative stress in turn can cause DNA damage, in the form of double strand breaks (DSBs). While the DNA damage repair (DDR) process functions to repair these DSBs, it is well established that with age, DDR is impaired and can no longer perform this function optimally. This leads to the induction of mutations and/or chromosomal aberration, which in turn can cause cell death, and, in extreme cases, cancer and neurodegenerative disorders [
2]. With aging there is a reduced ability to cope with cellular stresses, causing the body to become more prone to a wide variety of pathologies [
6]. The central nervous system (CNS), comprised of post-mitotic tissue, is profoundly affected by DDR deficiencies. DDR dysfunction in mature neural tissues is linked to both premature aging and neurodegenerative disorders, such as AD [
7].
Aging as a natural pathological process is slowly developing and coordinated by the interaction of multiple signaling systems across several somatic tissues. This complexity makes it a difficult process to therapeutically target. Chadwick et al. [
2] demonstrated that such complex systems possess some degree of organization, with some proteins possessing a greater regulatory network function than others. These are the so-called ‘keystones’ (alternatively termed ‘hubs’). Targeting these proteins facilitates regulating these complex disorders, in contrast to controlling the process at every molecular point. One such keystone recently identified is GIT2 (G protein-coupled receptor kinase interacting transcript 2), an ADP-ribosylation factor GTPase-activating protein (Arf-GAP), and a class A G-protein-coupled receptor (GPCR) interacting protein [
2,
8,
9]. GIT2 was identified as an important protein linked to several aspects of the aging process, through latent semantic indexing (LSI). As GIT2 is a potentially important keystone in aging, it might represent a crucial therapeutic target. However, canonical therapeutic targets are receptors, ion channels, kinases, and phosphatases, hence GIT2, being a scaffolding protein, does not represent an effective druggable target [
10]. It was recently demonstrated that, in addition to regulating intermediary cell metabolism events such as calcium mobilization, GPCRs can also effectively regulate the expression profiles of multiple signaling proteins via slower signaling modalities outside of the traditional G-protein-dependent functions [
11]. This suggests that GPCRs can be used to regulate the expression of specific signaling proteins, to improve therapeutic activity [
1,
9]. GPCRs are also interesting drug candidates due to their high diversity, targetability, and involvement in nearly every physiological process. Our ongoing research has also demonstrated that the signaling functions of these receptors are far more nuanced than previously conceptualized [
2,
10]. This signaling complexity facilitates the creation of novel, signal-selective GPCR therapeutics. Previous research, using GIT2 knock-out (KO) mice to investigate expression relationships in the context of metabolic aging, identified a consistently downregulated GPCR, the relaxin family peptide receptor 3 (RXFP3), in the CNS, pancreas, and liver [
10]. This association therefore suggests perhaps that GIT2 may act as a novel aging-specific signaling adaptor for the RXFP3 receptor.
1.2. Relaxin-Family Peptide Receptor 3
RXFP3, previously known as GPCR135, was deorphanized through the identification of its endogenous ligand relaxin-3 (RLN3), also known as insulin-like peptide 7 (INSL7). This class A rhodopsin-like receptor, together with its relaxin peptide family and their receptors, is a branch of the insulin superfamily, which consists of insulin and insulin-like growth factor 1 and 2 (IGF1 and -2) [
12]. This receptor, originally named the SALPR (somatostatin- and angiotensin-like peptide receptor [
13]), is primarily expressed in the CNS [
14,
15,
16]. There are currently four members in the relaxin family of GPCRs, i.e., RXFP1-4. In contrast to RXFP1 and RXFP2, RXFP3 and its closely related family member RXFP4 couple to Gαi, causing inhibition of cAMP production through a pertussis toxin-sensitive mechanism [
12]. RXFP3 and RXFP4, also resemble each other in structure, where both are classical type I peptide receptors with short amino (N)-terminal domains, and both are evolutionarily related to somatostatin and angiotensin receptors. In addition, the endogenous ligands for these two receptors are RLN3 and insulin-like peptide 5 (INSL5), respectively, which both play a role in neuroendocrine signaling [
17].
While RXFP3 has been classified as a class A rhodopsin-like receptor, it appears that it is not in its entirety a canonical rhodopsin-like GPCR. As detailed by van Gastel et al. [
10], RXFP3 does not contain a typical transmembrane domain 3 (TM3) Aspartate-Arginine-Tyrosine ‘DRY’ motif, but instead has a Threonine-Arginine-Tyrosine ‘TRY’ motif. This natural variation of this classical GPCR activation motif may demonstrate altered activation state kinetics, with an augmented level of ligand-independent constitutive activity. Furthermore, the highly conserved ExxxD motif, which is vital for RLN3 binding, has been identified at the second transmembrane domain on the extracellular side [
18].
The relaxin peptides are small (approximately 60 amino acids long), and similarly to insulin, share a common two-domain structure with an α- and a β-chain in their mature form [
12]. The α-chain appears to be important for receptor–ligand binding affinity, while the β-chain of RLN3 is mainly responsible for binding and activation of RXFP3 [
19]. RLN3 is the most recently identified relaxin family peptide, with the presence of the characteristic RxxxRxxI/V relaxin-binding motif found in the β-chain of all relaxin peptides; however, the remainder of the sequence displays low homology with other relaxin-family peptides. RLN3 is the only member of the relaxin-family with a sequence conserved across species [
20,
21], and this neuropeptide is believed to be the ancestral peptide of the family [
20,
22]. The RLN3/RXFP3 system demonstrates strong indications of ligand-receptor co-evolution, where nearly all amino acids have been subject to purifying selection for both genes and display a near perfect parallel in both mammals and teleosts [
23], both in structure and function [
20,
23]. Teleosts possess two rln3 paralogs (rln3a and b) and multiple rxfp3-type genes, which are not all orthologous to mammalian RXFP3 [
23]. However, it has been shown that intracellular loops 1 and 3 are important in terms of selection, indicating that a large part of selection for these GPCRs concerns downstream receptor signaling and not just selection for ligand binding [
23].
Further investigation of the functions of this receptor has uncovered that RXFP3 might play a vital role in several aging-related disorders, as a connection has been found to several hallmarks of aging, such as oxidative stress and DNA damage response [
24], similarly to the aging keystone GIT2 [
6,
7]. In addition, research by other groups has elucidated possible roles for RXFP3 in stress responses [
25], anxiety [
26], depression [
26,
27], feeding [
15,
28,
29,
30], arousal [
28], and alcohol addiction [
31]. Given the plethora of possible physiological activities of RXFP3, we will next assess how RXFP3 functionality may intersect with several of the classical hallmark processes involved with the aging process (
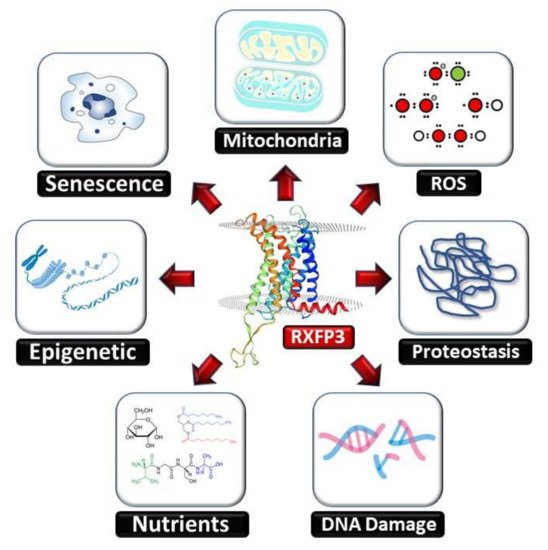
Figure 1).
Figure 1. The human RXFP3 receptor functionally intersects with multiple hallmarks of aging. The RXFP3 receptor has been shown by multiple researchers to be associated, at the molecular signaling level, to activities that constitute many of the classical hallmarks of aging. In doing so the RXFP3 potentially represents, in conjunction with its synergistic relationship with the GIT2 signaling adaptor, a novel systems-level therapeutic target for the multidimensional interdiction of the pathological aging process.
2. RXFP3 in Aging-Related Disorders
The aging process is largely driven by glucometabolic dysfunction. Hence, conditions such as T2DM or MetS are potent triggers of multiple forms of aging-related disease. Therefore, it is important to consider that the mechanisms through which glucose metabolism becomes dysregulated over time require more in-depth investigation. While the insulinotropic system is the primary mechanism for controlling glucose uptake and use, it has been shown in recent years that there are many other receptor systems (especially GPCRs) that also potently regulate glucose metabolism [
86,
128,
134,
135,
136,
137]. Here, we propose that the RXFP3 system, especially when it is actively interacting with GIT2, also forms part of this glucometabolic family [
2,
5,
24,
133]. Even though there has been a significant focus on investigating the role of glucose metabolism in the aging process (potentially via the profound link with mitochondrial support in aging), there is also the strong impact of the adipose tissue system in this paradigm [
138,
139,
140]. Reinforcing the potential importance of RXFP3 in the aging process, it has been demonstrated that RLN3 can play an important role in adipogenesis and maturation [
141]. This functionality may not be entirely unexpected as RXFP3 appears likely to be a manager of energy metabolism in times of aging-associated metabolic disruption [
10]. Hence, RXFP3 has been associated with the functionality of energy metabolic systems involving dietary-related weight gain, insulinotropic functions, and adipogenic activities that are strong players in the aging process [
3,
10,
29,
138,
142,
143,
144]. In the next sections we will highlight the contributions and activities of RLN3/RXFP3 signaling in aging-related disease. These insights strengthen the concept that the RXFP3-GIT2 signaling system may represent a novel signaling relationship system that can be developed for novel and effective anti-aging therapeutics.
2.1. Alzheimer’s Disease
It is now well appreciated that many classical central nervous system neurodegenerative disorders, such as Alzheimer’s, Parkinson’s, and Huntington’s disease, share many common etiological features with perhaps the primary one being metabolic dysfunction [
126,
128,
145,
146]. With respect to AD, it has been shown that RXFP3 levels are significantly altered in the neocortex of depressed Alzheimer’s patients [
147]. Alzheimer’s disease is primarily represented as a dysfunctional capacity for short-term memory formation and then, at a later stage, a dysfunction in long-term memory recollection. It is relevant to note that depletion of RXFP3 levels in the brain have been associated with long-term memory regulation in adult mice [
27]. In addition to long-term memory recall, RXFP3 functionality has also been associated with spatial memory formation [
148,
149].
2.2. Anxiety and Post-Traumatic Stress Disorder
Anxiety, along with associated disorders such as post-traumatic stress disorder (PTSD), have in recent years been strongly associated with premature aging conditions [
150,
151,
152,
153]. With respect to the impact of RXFP3 upon anxiety-related disorders, it has been shown that specific central stimulation generates an anxiolytic effect in model organisms [
154]. While acute effects of RXFP3 stimulation can generate anxiolytic effects, it has recently been shown that chronic localized RXFP3 stimulation instead can actually promote anxiety behavior [
26]. Thus, it appears that the anxiety-related activities of RXFP3 may be highly context specific in experimental animal models [
155]. Such a phenomena therefore entails a more detailed view in human patients of this specific situation and therefore RXFP3-based interventions could potentially be therapeutic targets for certain forms of anxiogenic activity.
It is interesting to note that the RXFP3-GIT2 signaling axis seems to be a priority signaling system for central anxiety/stress conditions, as not only is GIT2 involved in anxiety-related behavior directly, it is also a potent regulator of the glucose metabolic system that intertwines with anxiety-related conditions [
156,
157]. Moreover, it has been shown that both RXFP3 and GIT2 are highly expressed in the amygdala [
123,
158,
159]. There is also evidence to suggest that through common activities related to stress responses RXFP3 and GIT2 together may contribute in a coordinated manner to interconnect anxiety behaviors [
158] and stress responses such as hyperphagia or binge-eating [
160,
161,
162]. This anxiety-related condition will likely then feed into the generation of metabolic dysfunction via metabolic or diabetic syndromes. While impulsive behavioral responses in response to stress are seen with food, there is also considerable evidence that this stress-induced activity also includes augmented alcohol seeking activity [
163].
2.3. Schizophrenia
Our recent work has begun to provide evidence for the aging-related control of schizophrenia and schizophrenia-related conditions [
164,
165,
166]. Relaxin ligands, as well as RXFP3 itself, have been suggested by some researchers to be implicated in schizophrenia-related conditions [
167,
168]. Again, demonstrating the metabolic basis of aging, it has been shown that relaxin-3, RXFP3, and RXFP4 polymorphisms have been linked to metabolic disruptions in patients treated with anti-psychotic medications [
169]. Schizophrenia and other affective conditions are typified by periods of mania and heightened activity states, and it has been demonstrated that cognitive arousal states can also be strongly affected by RXFP3 activity in experimental animal models [
144]. In regard to the potential for a specific RXFP3-GIT2 signaling axis in the aging process, it is interesting to note that epigenetic modifications (hypermethylation) of GIT2 have recently been shown by the creation of schizophrenic differential methylation networks (SDMNs) from schizophrenia patient data [
170]. The specific effects of this modification of GIT2 in this paradigm has, however, yet to be shown [
164].
2.4. Obesity and Metabolic Dysfunction
Multiple experimental animal and longitudinal studies have demonstrated that diet-induced obesity promotes pro-aging phenotypes [
84,
97,
171,
172]. A considerable component of the obesity-based drive of aging likely results in alterations in insulin sensitivity as well as the drive towards alternative sources of energy, such as lipid- or protein-mediated metabolism that can incur a greater level of oxidative stress [
173,
174,
175].
RXFP3 expression and activity has been shown to be closely associated with both eating behavior alterations [
161,
176] as well as the physiological responses to augmented food intake [
160,
177,
178,
179]. In many of these experiments it has been noted that the role of RXFP3 in these scenarios is more pronounced in females compared to males [
177]. Concordant with this, it has been shown that female RXFP3 knock-out mice present with more heightened anxiety behavior than male RXFP3 knock-out mice in assessments of anxiety, such as the elevated plus maze. Hence, male RXFP3 knock-out mice spent more time in the open arms of the maze indicating their lower levels of anxiety than their female RXFP3 knock-out counterparts [
144]. Experimental animals fed a high fat/glucose diet (a common mechanism to accelerate metabolic aging) displayed significant alterations in the CNS expression of RLN3 and RXFP3 [
178]. These diet-induced obese (DIO) male rats displayed significantly higher levels of RLN3 expression compared to control diet ad libitum fed animals. This increased expression of RLN3 in DIO rats likely engenders the hyperphagic condition found in this experimental cohort. This study found that during a metabolic challenge of refeeding after food deprivation, the DIO rats only exhibited an increased expression of RXFP3 receptors in brain regions involved in food intake regulation [
178]. With respect to the links between the RLN3/RXFP3 system and human obesity it has been shown that RLN3 genetic polymorphisms are significantly associated with traits including obesity, hypercholesterolemia, and diabetes [
169]. The intersection of RLN3/RXFP3 signaling between stress-responsive binge eating and this greater role of RXFP3 in predisposition to obesity demonstrates the importance of this system in the control of glucometabolic dysfunction in the aging context. Given these associations, considerable activity has since focused on the development of RLN3-based interventions for obesity paradigms [
162,
179,
180].
2.5. Ischemic Stroke
Aging is considered to be one of the strongest independent risk factors for ischemic stroke-based injuries [
181,
182]. Hence, almost three-quarters of all strokes occur in people aged ≥65 years. Ischemic damage is cellular destruction associated with altered nutrient or oxygen support–resulting in energy deprivation and ROS-based damage. A recent study reported that relaxin peptides can protect tissues from ischemic damage. Using a rat stroke model, it was demonstrated that RXFP3 activation (using RLN2 and RLN3) reduced the extent of cellular/tissue damage induced by the application of vascular ligation [
53]. In this study it was reported that the ability to reduce the size of infarcts induced by transient middle cerebral artery occlusions was primarily mediated by selective activation of RXFP3. In addition to this, RXFP3 stimulation also demonstrated the capacity to reduce the damaging effects of oxygen and glucose deprivation in cellulo-cultured primary astrocytes.
2.6. Reproductive Aging
The control of reproductive behavior is tightly linked to the functional cellular/tissue mechanisms associated with energy metabolism and food availability [
59,
98,
122,
183,
184,
185]. As reproductive behavior and physiology are tightly controlled at certain points in the lifespan, it is not surprising that the broader relaxin system likely intersects with this aging–reproduction nexus. The role of relaxin in the reproductive process is one of the best studied aspects of its molecular biology [
186,
187,
188]. In a recent study that investigated the effects of premature defects in the female reproductive system (i.e., ovariectomy) it was found that in areas of the brain that demonstrated a dysfunctional network connectivity, there was a significant alteration in the levels of both RXFP3 expression and its potential preferred partner, GIT2 [
122]. Thus, it is likely that this receptor system [
10] can also form a functional bridge between the aging process and the reproductive system.
2.7. Alcohol Abuse
Alcohol use disorders are a leading cause of preventable deaths worldwide. Sobering patients often experience alcohol use relapses in times of physical and psychosocial stress. Both RLN3 and RXFP3 have been shown to modulate stress-induced relapse to alcohol seeking in rats. The amygdala is one of the most crucial areas of the CNS that controls this pathobiology. The central nucleus of the amygdala (CeA) in the rat receives a RLN3 innervation and possesses considerable levels of RXFP3 expression. In addition to this, the CeA receives considerable input from corticotropin releasing factor (CRF) neurons demonstrating a functional intersection between stress and this activity of the RLN3/RXFP3 system. In this specific scenario it is thought that CeL (lateral CeA) CRF neurons provide both local inhibitory GABA and excitatory CRF signals to the CeA neurons [
189].
As discussed previously, alcohol seeking behavior can be a major component of PTSD/anxiety phenotypes [
190]. Recent research has also indicated that the response to alcohol intake is also affected by the age of the afflicted individual [
191]. As we have contended that these stress-related conditions are potentially driven by metabolic disruption, it is unsurprising that recent research has started to propose that alcoholism behavior is also linked to pathological aging [
192,
193,
194]. Recent evidence has indicated that alcoholism can even lead to Alzheimer-like conditions that have a strong neuroinflammatory component [
195]. Excessive and inappropriate alcohol abuse results in the generation of multiple co-morbidities, including neurodegenerative atrophy, dysfunctional immune responses, and accelerated or premature aging [
194,
196]. One of the better studied functions of RXFP3 has been its regulatory capacity in alcohol seeking behavior [
26,
163]. In contrast to the RXFP3-based actions on feeding behavior [
177], it has been shown that only male RLN3 knock-out animals showed an increase in alcohol preference [
197]. Thus, it is possible that RXFP3-based therapeutics could be a potential future target for the treatment of alcoholism.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23084387