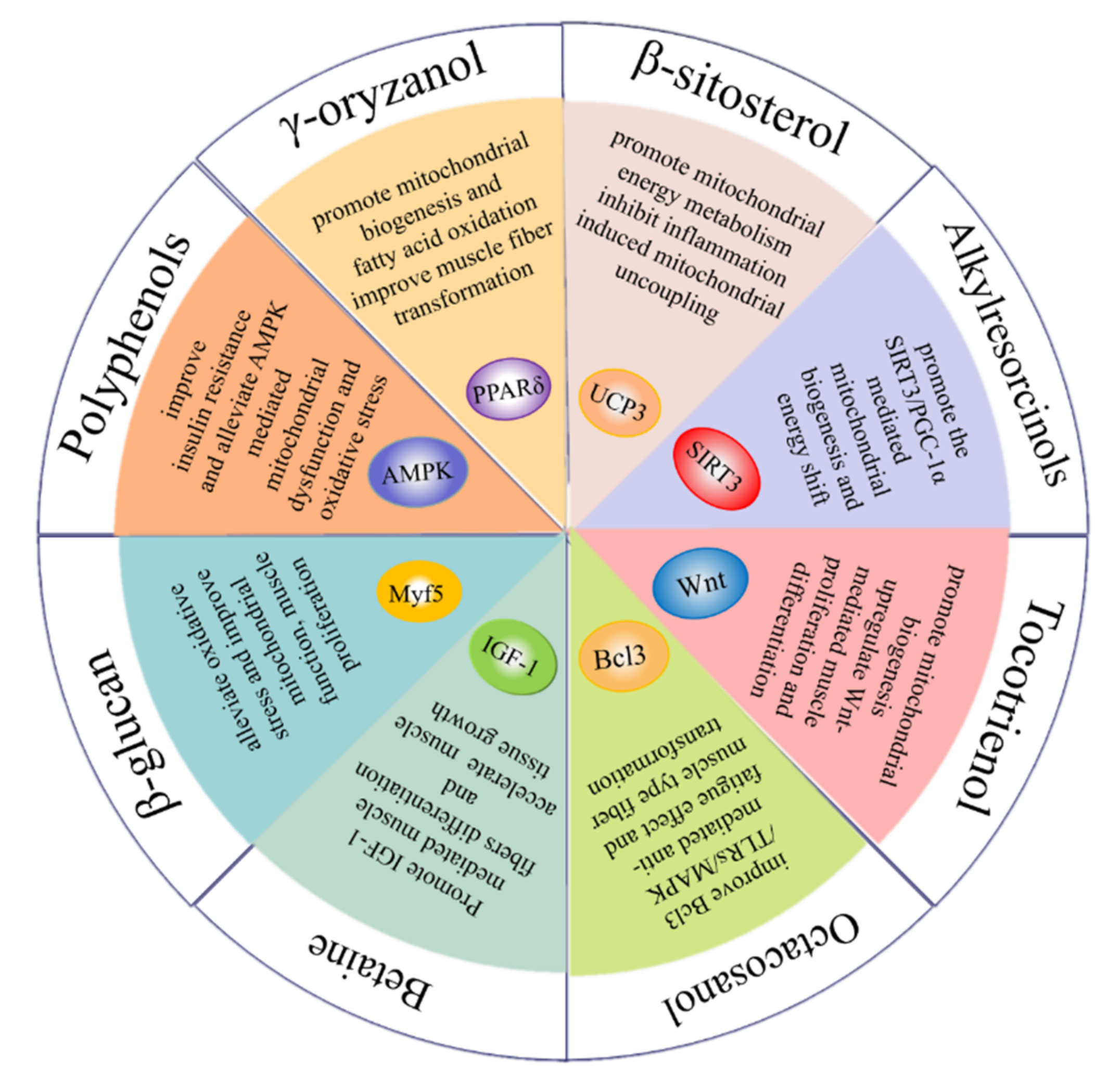
1. Whole Grain Bioactive Compounds in the Regulation of Skeletal Muscle Function
2. Phenolic Compounds
2.1. Phenolic Acids
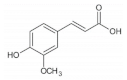
Ferulic acid is a phenolic acid widespread in whole grains such as oat [
39], wheat [
40], rice [
41], quinoa [
42] and corn [
43], which is evidenced to possess an impressive effect on modulating muscle function. It has been reported to prevent insulin resistance in skeletal muscle [
44]. It can also promote glucose uptake and regulate fatty acid oxidative decomposition in a dose-dependent manner, promoting redox balance in rat muscle [
5]. By triggering the PI3K/CpkC pathway, ferulic acid is proved to promote glucose uptake and glycogen synthesis, protecting against insulin resistance in L6 myotubes [
45]. After 30 days of dietary ferulic acid supplementation, the number and CSA of muscle fiber are enhanced in a zebrafish model. Ferulic acid also accelerated the muscle tissue growth of zebrafish by upregulating the expression of MyoD and myogenin [
46]. In addition, ferulic acid is proven to modulate PGC-1α-activated mitogenesis and energy metabolism via upregulating the expression of NRF1, TFAM and TFB2M [
47]. In C2C12 cells, ferulic acid exerts an anti-fatigue function via increasing succinate dehydrogenase (SDH) activity and decreasing lactate dehydrogenase (LDH) activity. Moreover, ferulic acid could stimulate the transformation from fast-twitch muscle fibers to slow-twitch muscle fibers via the SIRT1/AMPK signaling pathway [
48]. This effect is further verified in a weaned piglets model, demonstrating that SIRT1/AMPK/PGC-1α signaling was involved in ferulic-acid-mediated muscle transformation and anti-fatigue function [
49]. This evidence demonstrates the positive effect of ferulic acid on muscle metabolism and function.
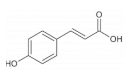
p-coumaric acid is widely distributed in whole grains including rice [
50], quinoa [
42], barley [
51] and wheat [
40]. It has been found that
p-coumaric acids possess the ability to regulate myogenic differentiation and lipid metabolism of skeletal muscle. For instance,
p-coumaric acid could increase the expression of myogenic regulatory factors myogenin and MyoD via activating AMPK signaling, improving myogenic differentiation of C2C12 cells [
52]. In the L6 cell model,
p-coumaric acid could increase fatty acid β-oxidation through stimulating ACC phosphorylation and the expression of PPARγ, preventing skeletal muscle against oxidative stress and inflammation caused by fatty acid accumulation [
53].
2.2. Resveratrol
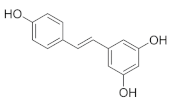
Resveratrol is a natural source of antioxidant and mitochondrial nutrients which can be found in whole grains, such as buckwheat [
54]. Resveratrol shows a positive effect on mitochondrial-dysfunction-induced muscle atrophy. For instance, in a dexamethasone-induced mouse model, resveratrol alleviates dexamethasone-induced mitochondrial dysfunction and muscle atrophy by blocking AMPK/FOXO3 signaling [
55]. In high-fat-diet-induced aged mice, muscle loss and myofiber size decrease are reversed by resveratrol supplementation. Resveratrol could improve the mitochondrial dysfunction and oxidative-stress-induced muscle atrophy via activation of the PKA/LKB1/AMPK pathway [
56]. Moreover, resveratrol has been reported to enhance muscle proliferation and differentiation in obese mouse muscle. The level of muscle regeneration proteins including MyoG, Myf5 and Pax7 is upregulated by resveratrol administration [
57]. In response to skeletal-muscle glucose metabolism disorders caused by obesity, resveratrol can reduce insulin resistance by increasing muscle glycogen synthesis and reducing ROS levels in high-fat-diet-fed mice [
58]. In the C2C12 model, resveratrol promotes AKT activation and glucose absorption, as well as decreased glutathione (GSH) level. These results suggest that resveratrol could alleviate muscle insulin resistance by modulating redox levels [
59]. Resveratrol is evidenced to alleviate TNF-α-induced muscle hypertrophy and muscle atrophy in C2C12 cells via inhibiting the atrophy-related ubiquitin ligase through upregulating AKT/mTOR/FOXO1 signaling [
60]. Furthermore, resveratrol displays impressive activity in promoting the transformation of muscle fiber types. The expression of myosin heavy chain (MyHC) 1, MyHC2a and MyHC2x in mouse muscle is increased with resveratrol supplementation, indicating the transformation from fast- to slow-twitch muscle fibers. This conversion is achieved by activating the AdiopR1-AMPK-PGC-1α signaling pathway [
61]. In C2C12 myotubes, resveratrol supplementation increased the activities of lactate dehydrogenase (LDH) and malate dehydrogenase (MDH) while it reversed the elevated level of miR-22-3p, suggesting its anti-fatigue effect via stimulating the transformation of muscle fiber from fast-twitch to slow-twitch type [
62]. In addition, resveratrol exerts an anti-fatigue effect in contusion-induced-injury mice, shown as the increased activity of lactate dehydrogenase (LDH) and creatine kinase (CK), displaying an important role in muscle metabolic regulation [
63].
2.3. Flavonoids
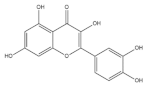
As a special subclass of flavonoids, quercetin is a natural bioactive compound abundantly present in common cereal grains, especially in buckwheat [
64], followed by rice [
50], quinoa [
42], corn [
43] and oat [
39]. Quercetin has displayed notable antioxidative and anti-inflammatory effects and shows potential benefits for muscle function [
65]. By increasing the expression of adiponectin, quercetin stimulates muscle-fiber-type transformation from fast-twitch to slow-twitch myofibers [
66]. It has been also demonstrated that quercetin increases slow-twitch myofibers by regulating AMPK/SIRT1 signaling [
67]. Heme oxygenase-1 (HO-1) serves as an essential factor to decrease the inflammatory response and oxidative stress. Mediated by an HO-1/NRF2-dependent mechanism, quercetin is found to alleviate obesity-induced muscle atrophy [
68]. In relation to the mitochondrial network, quercetin has been shown to significantly improve mitogenesis in denervated mice, through which muscle atrophy is improved [
69]. Moreover, it is shown that quercetin ameliorates TNF-α-induced skeletal muscle insulin resistance in C2C12 cells [
70]. The anti-fatigue function of quercetin might be related to its ability to scavenge free radicals [
71]. Overloaded exercise causes excessive ROS generation, which further leads to mitochondrial dysfunction and muscle protein loss [
72]. In a BALB/c mouse model, quercetin displayed obvious fatigue resistance activity, shown as enhanced muscle glycogen content, elevated mitochondrial fatty acid β-oxidation and decreased oxidative stress [
73]. These findings demonstrate the impressive effect of quercetin on improving muscle mitochondrial function and alleviating the inflammatory reaction in the muscle.
Oligomeric procyanidins (OPCs) are mainly found in dark-colored grains, such as black rice and red rice [
74]. As reported, OPCs could effectively inhibit glucose metabolism disorder in muscle tissue caused by diabetes. OPCs are shown to improve lipid metabolism by inhibiting mTOR signaling and enhancing glucose homeostasis and insulin sensitivity in the skeletal muscle of diabetic mice [
75]. In obese mice, OPC supplementation increases glycogen synthesis and glucose uptake, exerting an antidiabetic effect in an insulin-independent manner [
76]. Moreover, oral OPC administration alleviates acute hyperglycemia and improves insulin sensitivity via promoting AMPK-signaling-mediated GLUT4 translocation in ICR mice [
77].
Cyanidin-3-glucoside (Cy3G) widely exists in pigmented whole grains, such as black rice [
50], purple corn [
43], oat, wheat and rye [
78]. In vivo and in vitro studies have shown that Cy3G possesses anti-obesity and antidiabetic effects [
79,
80]. It has been demonstrated that as effective radical scavengers, Cy3G exerts an anti-diabetes effect via reducing oxidative stress in human skeletal muscle cells [
81]. In an ICR mouse model, oral supplementation with Cy3G shows improved exercise endurance, performed as weight-loaded swimming time. Moreover, the increased level of lactic acid accumulated after excessive exercise is reversed by Cy3G via activating PGC-1α signaling, indicating that Cy3G exerts its anti-fatigue effect through modulating the PGC-1α-mediated lactic acid metabolism [
82]. Consistent with the in vivo data, Cy3G could also upregulate PGC-1α expression in C2C12 myotubes, improving muscle metabolic function [
82].
Catechins are flavanols widely found in whole grains such as wheat [
83], barley and buckwheat [
84]. The isomers of catechins including catechin, epicatechin (EC) and epigallocatechin gallate (EGCG) are proved to increase muscle mass and muscle strength, enhancing the exercise endurance of rats [
85]. Catechins could upregulate the expression of Myf5 in muscle stem cells via activating Akt phosphorylation and thus improve muscle regeneration [
86]. In the C2C12 cell model, catechin supplementation was found to promote myotube differentiation, shown as an increased level of MyoD, MyoG and MyHC, suggesting its improvement effect on skeletal muscle regeneration and repair [
87]. Moreover, catechins have shown impressive modulation of the promotion of mitochondrial function in skeletal muscle. In an LCR (low running capacity) rat model, EC treatment improved mitochondrial biogenesis and respiratory capacity via activating p38 MAPK signaling in the skeletal muscle, enhancing muscle resistance to fatigue [
88]. Using a diabetic rat model, EGCG supplementation could alleviate diabetes-induced mitochondrial dysfunction in skeletal muscle, characterized by increased expression of PGC-1α, MFN2 and COXⅠ. The improvement effect of catechins is possibly achieved by promoting mitochondrial autophagy via activating the ROS-ERK/JNK-p53 pathway [
89]. Moreover, EGCG could target Wnt signaling and increase the level of downstream MyoD, promoting muscle regeneration [
90]. In a rat model of sarcopenia, EGCG administration could increase skeletal muscle mass which is presumably attributed to the downregulated muscle protein degradation mediated by the ubiquitin–proteasome system (UPS) and the upregulated muscle protein synthesis mediated by IGF-1 [
91]. Moreover, EGCG inhibits the expression of oxidatively modified protein induced by electrical stimulation in rat skeletal muscle, preventing the muscle from oxidative damage [
92]. These data suggest that catechin could facilitate skeletal muscle function through promoting myotube differentiation, enhancing mitochondrial function and defending oxidative stress.
Rutin is a flavonol glycoside and is widely distributed in whole grains such as buckwheat [
84], wheat [
93] and quinoa [
94]. The antioxidant and anti-inflammatory properties of rutin are demonstrated from both in vivo and in vitro studies [
95]. The decreased mitochondrial number and mitochondrial biogenesis in skeletal muscle are associated with obesity [
96]. In an obese rat model, rutin supplementation significantly enhances mitochondrial DNA (mtDNA) content and mitochondrial biogenesis in skeletal muscle by activating AMPK signaling. The expression levels of PGC-1α, NRF1, TFAM and SIRT1 are also increased, indicating that rutin could restore obesity-induced skeletal muscle mitochondrial dysfunction [
97]. Using a forced swimming mouse model, rutin administration was proved to upregulate PGC-1α-mediated mitochondrial biogenesis and decrease the level of lactic acid in skeletal muscle, improving the fatigue-resistance capacity of mouse muscle [
98]. In addition, rutin was found to inhibit oxidative stress and inflammation-induced muscle injury. Specifically, rutin intervention improves the inflammatory state by inhibiting NF-κB signaling, manifested in the decreased expression of IL-6 and iNOS, preventing muscle injury of C2C12 cells [
99].
3. Carotenoids
Carotenoids are lipid-soluble phytochemicals and the inactive precursor of vitamin A [
100], which are abundantly distributed in corn, wheat and barley [
101]. As the main component of carotenoids, lutein is reported to improve muscle function via alleviating oxidative stress [
102]. In a rat model of ischemia-reperfusion (IR) muscle injury, lutein supplementation decreases the production of ROS and the expression of COX-2 by inhibiting NF-κB signaling, protecting against skeletal muscle IR injury [
103]. These observations imply the muscle improvement benefit of lutein presumably via its antioxidant and anti-inflammatory effect.
β-carotenes are the most ubiquitous and stable natural pigments in nature [
104]. β-carotene supplementation could enhance muscle mass by promoting IGF-1-mediated muscle protein synthesis and reducing ubiquitin–protease-mediated muscle protein degradation in an atrophy mouse model [
105]. Atrogin-1 and MuRF1 are identified as muscle-specific ubiquitin ligases that are upregulated in skeletal muscle under atrophy-inducing conditions [
106]. β-carotene treatment is reported to increase muscle mass and decrease the level of Atrogin-1 and MuRF1 in denervated mice, exhibiting a promotion effect on oxidative-stress-induced muscle atrophy [
107].
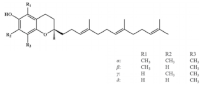
4. Tocotrienol
Tocotrienol, the unsaturated form of vitamin E, mainly exists in buckwheat [
108], rice, rye and oat [
39]. Tocotrienol receives attention due to its unique biological properties such as antioxidant and anti-inflammatory activity [
109]. Increasing evidence has shown that tocopherol exhibits a positive effect on maintaining muscle function. It has been proven that dietary supplementation of tocotrienol improves muscle atrophy and insulin resistance in type 2 diabetic mice. This effect is achieved by activating AMPK/SIRT1/PGC-1α signaling and increasing mitochondrial biogenesis in the skeletal muscle, indicating that tocotrienol improves muscle disorder via upregulating mitochondrial function [
110]. In addition, muscle differentiation and regeneration are essential for protecting against aging-related loss of muscle mass and strength. Tocotrienol treatment is found to increase the expression of myoblast-differentiation-related proteins such as MyoD, MyoG and MRF4. Moreover, tocotrienol increased the miR-206 level in human skeletal muscle myoblasts (HSMMs), which resulted in an increased expression of IGF1R and decreased expression of Pax7, promoting muscle cell differentiation [
111]. Through transcriptomic analysis, tocotrienol was found to promote muscle cell regeneration via Wnt signaling and alleviate aging-related muscle loss through the FOXO pathway [
112]. Additionally, Chung et al. reported that tocotrienol administration improved insulin intolerance and increased soleus muscle weight of obese mice, indicating its potential to ameliorate muscle dysfunction [
113].
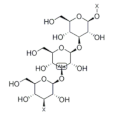
5. β-Glucan
β-glucan is a soluble dietary fiber which exists abundantly in oat [
39], rice [
114], wheat, barley and rye [
108]. β-glucan has been evidenced to have a positive effect on muscle health [
115]. For example, dietary supplementation with oat β-glucan is found to decrease the activity of lactate dehydrogenase and creatine kinase in serum, while it increases the glycogen content in the gastrocnemius muscle of rats. These results indicate that oat β-glucan facilitates the recovery of trained rats from fatigue [
116]. In addition, β-glucan is found to promote the transformation of fast-twitch muscle fibers to slow-twitch type in the C2C12 model. Moreover, the levels of myoblast proliferation markers such as Myf5 and Mox2 are increased by β-glucan treatment, suggesting the anti-fatigue effect via improving muscle fiber proliferation and transformation [
117]. Mitochondria are responsible for providing energy during immune action and the enhancement of mitochondrial function is conducive to preventing oxidative stress. In the Duchenne muscular dystrophy (DMD) zebrafish model, β-glucan intervention improves mitochondrial respiration and zebrafish exercise capacity, suggesting that β-glucan could alleviate the symptoms of DMD through regulating mitochondria function [
118].
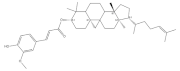
6. γ-Oryzanol
γ-oryzanol is a mixture of feruloyl esters with triterpenol as the main component and is mainly found in rice, corn and wheat [
119,
120]. γ-oryzanol supplementation has been evidenced to improve exercise performance such as grip strength and running time in aged mice. Additionally, γ-oryzanol has been found to promote the formation of slow-twitch muscle fiber, which is a fatigue-resistant myofiber type and conductive to the prevention of muscle aging. By binding to PPARδ and ERRγ directly, γ-oryzanol could upregulate mitochondrial biogenesis and alleviate inflammation, reducing the occurrence of muscle weakness of the aged mice [
121]. Moreover, γ-oryzanol supplementation is demonstrated to promote GLUT4 translocation, alleviating insulin resistance in obese mice [
122]. In addition, the antioxidant function of γ-oryzanol in muscle could be enhanced under an exercise condition [
123], suggesting the physical exercise combined with γ-oryzanol supplementation might be a beneficial strategy to prevent muscle dysregulation.
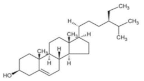
7. β-Sitosterol
As the most abundant phytosterol, β-sitosterol exists widely in plant products such as barley [
51], wheat, oat and quinoa [
39,
124]. It is considered to be a mild free-radical scavenger and has positive effects on maintaining cell membrane stability, as well as inhibiting inflammation and Alzheimer’s disease [
125]. In ICR mice, β-sitosterol promotes mitochondrial electron transport and mitochondrial oxidative phosphorylation, enhancing muscle strength. In addition, β-sitosterol promotes the energy metabolism of C2C12 cells by activating UCP3 and inducing mitochondrial uncoupling [
126]. Meanwhile, β-sitosterol dramatically enhanced mitochondrial biogenesis in chicken skeletal muscle via activating the PGC-1α/TFAM pathway [
127]. In an L6 cell model, β-sitosterol could promote glucose uptake and lipid metabolism by activating ACC phosphorylation. A decreased level of triglycerides and cholesterol is achieved with LKB1-mediated AMPK activation [
128]. Likewise, β-sitosterol supplementation is proved to facilitate GLUT4 translocation and upregulate insulin sensitivity in HFD-induced diabetic rats, manifested in the increased activity of glycolytic and gluconeogenesis enzymes [
129]. Taken together, these data suggest the promoting effect of β-sitosterol on mitochondrial function and glucose metabolism in the skeletal muscle.
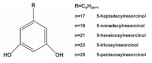
8. Alkylresorcinols
Alkylresorcinols (ARs) are phenolic lipids and exist abundantly in the bran part of cereal grains such as wheat, barley and rye [
108]. They consists of various homologues distinguished by the length of the alkyl chain (C17:0−C25:0) attached to the phenyl ring [
3]. ARs derived from wheat bran extracts have been indicated to prevent muscle atrophy in denervated mice via regulating the disruption of fatty acid metabolism induced by lipid autophagy [
4]. In addition, dietary supplementation containing ARs was found to protect against isoproterenol-induced myofibrillar degeneration in rats via inhibiting oxidative damage caused by lipid accumulation [
130]. 5-heptadecylresorcinol (AR-C17) as the main homologue of ARs has been proven to have the best anti-inflammatory effect among the identified ARs homologues in our previous study [
3]. We found that AR-C17 alleviated muscle dysfunction via upregulating SIRT3/PGC-1α-mediated mitochondrial function. The mitochondria content and mitochondrial-biogenesis-related proteins such TFAM and NRF1 were significantly increased, contributing to enhanced exercise performance [
131].
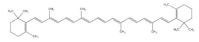
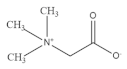
9. Betaine
Betaine is a natural antioxidant widely present in common cereals including whole grain wheat, barley, rye and oat [
132]. Betaine supplementation is reported to improve muscle strength and power [
133]. IGF-1 stimulates myotube growth and differentiation by activating the expression of downstream MyoD and myogenin. Betaine is reported to increase the expression of IGF-1 in murine myoblasts [
134]. AKT is considered as a key regulator of muscle signaling and protein synthesis. Additionally, betaine supplementation could increase anabolism and weaken catabolism in muscle tissue by stimulating IGF-1/AKT signaling [
135]. In an in vitro C2C12 model, betaine supplementation promoted myotube differentiation by upregulating the secretion of miR-29b-3p. In addition, through the activation of NFATc1/MyoD signaling, betaine promotes the transformation from fast-twitch to slow-twitch myofibers [
136]. Moreover, betaine could promote muscle energy metabolism by upregulating mitochondrial biogenesis, accompanied by increased glucose consumption and ATP production in C2C12 cells [
137].
10. Octacosanol
Octacosanol is a primary aliphatic alcohol that is abundant in rice [
138] and wheat [
139]. According to literature data, octacosanol has been identified as an anti-fatigue agent which could be stored in skeletal muscle after serial doses administration [
140]. In a trained rats model, dietary supplementation with octacosanol also showed a better physical recovery after exhaustion and there was a shift from fast-twitch to slow-twitch myofibers in skeletal muscle [
141]. In addition, orally administrated octacosanol might be converted into fatty acids and related to energy utilization, indicating its potential to improve exercise capacity through upregulating the energy supply [
142]. The underlying mechanism of the anti-fatigue effect by octacosanol might be through increasing the expression of Prx and decreasing the expression of Trim63 in trained mice. Based on the gene ontology analysis, this improvement might be mediated by the Bcl3/TLRs/MAPK signaling pathway [
143]. Overall, octacosanol resists fatigue possibly via modulating muscle-fiber-type transition and muscular energy metabolism.
Table 1. The structure and content of bioactive components in whole grains.