The use of fossil energy sources has a negative impact on the economic and socio-political stability of specific regions and countries, causing environmental changes due to the emission of greenhouse gases. Moreover, the stocks of mineral energy are limited, causing the demand for new types and forms of energy. Biomass is a renewable energy source and represents an alternative to fossil energy sources. Microorganisms produce energy from the substrate and biomass, i.e., from substances in the microenvironment, to maintain their metabolism and life. However, specialized microorganisms also produce specific metabolites under almost abiotic circumstances that often do not have the immediate task of sustaining their own lives. Microorganisms can change the current paradigm, energy–environment, and open up countless opportunities for producing new energy sources, especially hydrogen, which is an ideal energy source for all systems (biological, physical, technological). Developing such energy production technologies can significantly change the already achieved critical level of greenhouse gases that significantly affect the climate.
- hydrogen
- microorganisms
- biofuels
- energy
1. Introduction
2. Microbial Technologies for Biofuel Production
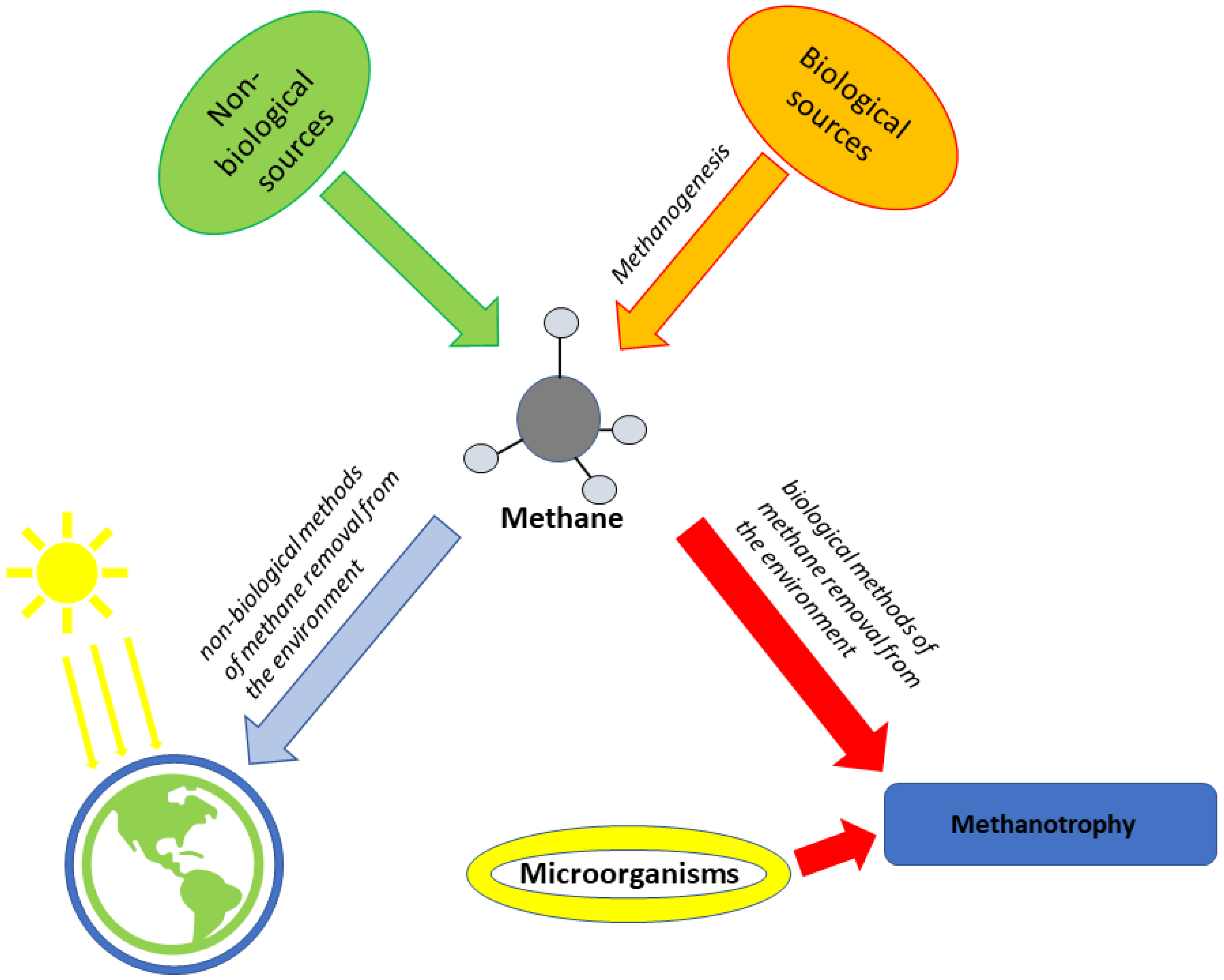
3. Future Perspective
| Biofuel | Advantage | Disadvantage | Ref. |
|---|---|---|---|
| Bioethanol | Renewable sources; low cost; algae can rapidly absorb carbon dioxide, accumulate high concentrations of lipid and carbohydrates, be easily cultivated, and require less land than terrestrial plants | High costs of lignocellulosic feedstock; inputs of energy and water; requirements for large volume bioreactors and distillation columns; generation of large volumes of waste or low-value coproducts | [37] |
| Biodiesel | Renewable, sustainable, environmentally friendly, and biodegradable sources; low cost and high conversion rate; ideal replacement for petrol; reducing greenhouse gases; less harmful carbon emission; ecologically and economically sustainable bioprocess; use of existing engines without changes | High energy consumption; environmentally unfriendly processing including chemical catalysts, high cost, and limited supply of feedstocks; complex production processes; downstream technology; simultaneously produced waste; production is dependent on large quantities of water and oil | [38] |
| Biohydrogen | Renewable sources; cleanliness; low greenhouse gas emissions; biohydrogen has the advantage of being able to use a wide range of substrates to produce hydrogen; the first stage of the waste treatment and valorization process uses mild temperatures and does not need the external addition of metal catalysts; clear environmental benefits | Low performance; high capital cost investment; expensive materials; complex maintenance; variable energy loss; decreasing hydrogen production with the increase in the volume of the reactor; hydrogen storage; global-warming potential; land use; terrestrial- and freshwater-ecotoxicity potential; ecotoxicity potential; human-toxicity potential | [39][40][41] |
This entry is adapted from the peer-reviewed paper 10.3390/en15176365
References
- Gupta, A.; Gupta, R.; Singh, R.L. Microbes and Environment. In Principles and Applications of Environmental Biotechnology for a Sustainable Future; Springer: Singapore, 2017; pp. 43–84.
- Hoppe, T.; Butenko, A.; Heldeweg, M. Innovation in the European Energy Sector and Regulatory Responses to It: Guest Editorial Note. Sustainability 2018, 10, 416.
- Agyekum, E.B.; Nutakor, C.; Agwa, A.M.; Kamel, S. A Critical Review of Renewable Hydrogen Production Methods: Factors Affecting Their Scale-Up and Its Role in Future Energy Generation. Membranes 2022, 12, 173.
- Teng, Y.; Xu, Y.; Wang, X.; Christie, P. Function of Biohydrogen Metabolism and Related Microbial Communities in Environmental Bioremediation. Front. Microbiol. 2019, 10, 106.
- Fabris, M.; Abbriano, R.; Pernice, M.; Sutherland, D.L.; Commault, A.S.; Hall, C.C.; Labeeuw, L.; McCauley, J.I.; Kuzhiumparambil, U.; Ray, P.; et al. Emerging Technologies in Algal Biotechnology: Toward the Establishment of a Sustainable, Algae-Based Bioeconomy. Front. Plant Sci. 2020, 11, 279.
- Sheldon, R.A. Biocatalysis and biomass conversion: Enabling a circular economy. Philos. Trans. R. Soc. A 2020, 378, 20190274.
- Óhaiseadha, C.; Quinn, G.; Connolly, R.; Connolly, M.; Soon, W. Energy and Climate Policy—An Evaluation of Global Climate Change Expenditure 2011–2018. Energies 2020, 13, 4839.
- Alzate, C.A.C.; Serna-Loaiza, S.; Ortiz-Sanchez, M. Sustainable Biorefineries: What was Learned from the Design, Analysis and Implementation. J. Sustain. Dev. Energy Water Environ. Syst. 2020, 8, 88–117.
- Waldrop, M.M. News Feature: Microbes for better sewage treatment. Proc. Natl. Acad. Sci. USA 2021, 118, e2112863118.
- Zetterholm, J.; Bryngemark, E.; Ahlström, J.; Söderholm, P.; Harvey, S.; Wetterlund, E. Economic Evaluation of Large-Scale Biorefinery Deployment: A Framework Integrating Dynamic Biomass Market and Techno-Economic Models. Sustainability 2020, 12, 7126.
- Janssen, P.; Lambreva, M.D.; Plumeré, N.; Bartolucci, C.; Antonacci, A.; Buonasera, K.; Frese, R.; Scognamiglio, V.; Rea, G. Photosynthesis at the forefront of a sustainable life. Front. Chem. 2014, 2, 36.
- Li, G.; Hu, R.; Wang, N.; Yang, T.; Xu, F.; Li, J.; Wu, J.; Huang, Z.; Pan, M.; Lyu, T. Cultivation of microalgae in adjusted wastewater to enhance biofuel production and reduce environmental impact: Pyrolysis performances and life cycle assessment. J. Clean. Prod. 2022, 355, 131768.
- Li, G.; Zhang, J.; Li, H.; Hu, R.; Yao, X.; Liu, Y.; Zhou, Y.; Lyu, T. Towards high-quality biodiesel production from microalgae using original and anaerobically-digested livestock wastewater. Chemosphere 2021, 273, 128578.
- Rather, R.A.; Wani, A.W.; Mumtaz, S.; Padder, S.A.; Khan, A.H.; Almohana, A.I.; Almojil, S.F.; Alam, S.S.; Baba, T.R. Bioenergy: A foundation to environmental sustainability in a changing global climate scenario. J. King Saud. Univ. Sci. 2022, 34, 101734.
- Directive (EU) 2018/2001 of the European Parliament and of the Council of 11 December 2018 on the Promotion of the Use of Energy from Renewable Sources (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/eli/dir/2018/2001/oj (accessed on 22 August 2022).
- Vučurović, D.; Bajić, B.; Vučurović, V.; Jevtić-Mučibabić, R.; Dodić, S. Bioethanol Production from Spent Sugar Beet Pulp—Process Modeling and Cost Analysis. Fermentation 2022, 8, 114.
- Nordahl, S.L.; Devkota, J.P.; Amirebrahimi, J.; Smith, S.J.; Breunig, H.M.; Preble, C.V.; Satchwell, A.J.; Jin, L.; Brown, N.J.; Kirchstetter, T.W.; et al. Life-Cycle Greenhouse Gas Emissions and Human Health Trade-Offs of Organic Waste Management Strategies. Environ. Sci. Technol. 2020, 54, 9200–9209.
- Tapio, I.; Snelling, T.J.; Strozzi, F.; Wallace, R.J. The ruminal microbiome associated with methane emissions from ruminant livestock. J. Anim. Sci. Biotechnol. 2017, 8, 7.
- Conrad, R. The global methane cycle: Recent advances in understanding the microbial processes involved. Environ. Microbiol. Rep. 2009, 1, 285–292.
- Buan, N.R. Methanogens: Pushing the boundaries of biology. Emerg. Top. Life Sci. 2018, 2, 629–646.
- Hook, S.E.; Wright, A.-D.G.; McBride, B.W. Methanogens: Methane Producers of the Rumen and Mitigation Strategies. Archaea 2010, 2010, 945785.
- Srivastava, P.; Marjo, C.; Gerami, A.; Jones, Z.; Rahman, S. Surface Analysis of Coal Indicating Neutral Red Enhances the Precursor Steps of Methanogenesis. Front. Microbiol. 2020, 11.
- Ney, B.; Ahmed, F.H.; Carere, C.R.; Biswas, A.; Warden, A.C.; E Morales, S.; Pandey, G.; Watt, S.J.; Oakeshott, J.G.; Taylor, M.C.; et al. The methanogenic redox cofactor F420 is widely synthesized by aerobic soil bacteria. ISME J. 2017, 11, 125–137.
- Grinter, R.; Greening, C. Cofactor F420: An expanded view of its distribution, biosynthesis and roles in bacteria and archaea. FEMS Microbiol. Rev. 2021, 45, fuab021.
- Cavicchioli, R.; Ripple, W.J.; Timmis, K.N.; Azam, F.; Bakken, L.R.; Baylis, M.; Behrenfeld, M.J.; Boetius, A.; Boyd, P.W.; Classen, A.T.; et al. Scientists’ warning to humanity: Microorganisms and climate change. Nat. Rev. Microbiol. 2019, 17, 569–586.
- Wang, Y.; Hu, P.; Yang, J.; Zhu, Y.-A.; Chen, D. C–H bond activation in light alkanes: A theoretical perspective. Chem. Soc. Rev. 2021, 50, 4299–4358.
- Meloni, E.; Martino, M.; Palma, V. A Short Review on Ni Based Catalysts and Related Engineering Issues for Methane Steam Reforming. Catalysts 2020, 10, 352.
- Wang, S.; Dames, E.E.; Davidson, D.F.; Hanson, R.K. Reaction Rate Constant of CH2O + H = HCO + H2 Revisited: A Combined Study of Direct Shock Tube Measurement and Transition State Theory Calculation. J. Phys. Chem. A 2014, 118, 10201–10209.
- Song, H.; Meng, X.; Wang, S.; Zhou, W.; Wang, X.; Kako, T.; Ye, J. Direct and Selective Photocatalytic Oxidation of CH4 to Oxygenates with O2 on Cocatalysts/ZnO at Room Temperature in Water. J. Am. Chem. Soc. 2019, 141, 20507–20515.
- Nisbet-Jones, P.B.R.; Fernandez, J.M.; Fisher, R.E.; France, J.L.; Lowry, D.; Waltham, D.A.; Maisch, C.A.W.; Nisbet, E.G. Is the destruction or removal of atmospheric methane a worthwhile option? Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2022, 380, 20210108.
- Islam, J.; Shrestha, N.; Bathi, J.R.; Sani, R.K.; Gadhamshetty, V. Surface Modification Approaches for Methane Oxidation in Bioelectrochemical Systems. In Bioelectrochemical Systems; Springer: Singapore, 2020; pp. 343–374.
- Thauer, R.K.; Kaster, A.-K.; Seedorf, H.; Buckel, W.; Hedderich, R. Methanogenic archaea: Ecologically relevant differences in energy conservation. Nat. Rev. Microbiol. 2008, 6, 579–591.
- Dlugokencky, E.J.; Nisbet, E.G.; Fisher, R.; Lowry, D. Global atmospheric methane: Budget, changes and dangers. Philos. Trans. R. Soc. London. Ser. A Math. Phys. Eng. Sci. 2011, 369, 2058–2072.
- De Souza, R.F.B.; Florio, D.Z.; Antolini, E.; Neto, A.O. Partial Methane Oxidation in Fuel Cell-Type Reactors for Co-Generation of Energy and Chemicals: A Short Review. Catalysts 2022, 12, 217.
- Kumar, R.; Kumar, P. Future Microbial Applications for Bioenergy Production: A Perspective. Front. Microbiol. 2017, 8, 450.
- Sahni, S.; Singh, M.K.; Narang, A. Sustainable Solution for Future Energy Challenges Through Microbes. Energy 2021, 231–249.
- Tse, T.J.; Wiens, D.J.; Reaney, M.J.T. Production of Bioethanol—A Review of Factors Affecting Ethanol Yield. Fermentation 2021, 7, 268.
- Wang, H.; Peng, X.; Zhang, H.; Yang, S.; Li, H. Microorganisms-promoted biodiesel production from biomass: A review. Energy Convers. Manag. X 2021, 12, 100137.
- Osman, A.I.; Mehta, N.; Elgarahy, A.M.; Hefny, M.; Al-Hinai, A.; Al-Muhtaseb, A.H.; Rooney, D.W. Hydrogen production, storage, utilisation and environmental impacts: A review. Environ. Chem. Lett. 2021, 20, 153–188.
- Park, S.-G.; Rajesh, P.; Sim, Y.-U.; Jadhav, D.A.; Noori, T.; Kim, D.-H.; Al-Qaradawi, S.Y.; Yang, E.; Jang, J.-K.; Chae, K.-J. Addressing scale-up challenges and enhancement in performance of hydrogen-producing microbial electrolysis cell through electrode modifications. Energy Rep. 2022, 8, 2726–2746.
- Germscheidt, R.L.; Moreira, D.E.B.; Yoshimura, R.G.; Gasbarro, N.P.; Datti, E.; dos Santos, P.L.; Bonacin, J.A. Hydrogen Environmental Benefits Depend on the Way of Production: An Overview of the Main Processes Production and Challenges by 2050. Adv. Energy Sustain. Res. 2021, 2, 2100093.
- Pulyaeva, V.N.; A Kharitonova, N.; Kharitonova, E.N. Advantages and Disadvantages of the Production and Using of Liquid Biofuels. IOP Conf. Ser. Mater. Sci. Eng. 2020, 976, 012031.
