Ultra-processed foods are ready-to-heat and ready-to-eat products created to replace traditional homemade meals and dishes due to convenience and accessibility. Because of their low-fiber and high-fat and sugar composition, these foodstuffs could induce a negative impact on health. They are partially responsible for obesity and chronic non-transmissible diseases; additionally, they could impact in the prevalence of autoimmune diseases such as type 1 diabetes and celiac disease. The rationale is that the nutritional composition of ultra-processed foodstuffs can induce gut dysbiosis, promoting a pro-inflammatory response and consequently, a “leaky gut”. These factors have been associated with increased risk of autoimmunity in genetically predisposed children. In addition, food emulsifiers, commonly used in ultra-processed products could modify the gut microbiota and intestinal permeability, which could increase the risk of autoimmunity. In contrast, unprocessed and minimally processed food-based diets have shown the capacity to promote gut microbiota eubiosis, anti-inflammatory response, and epithelial integrity, through bacterial butyrate production.
- ultra-processed food products
- autoimmunity risk
- type 1 diabetes
- celiac disease
- microbiota
1. Introduction
2. Microbiota, Gut Health and Autoimmunity
3. Newer Is Not Always Better
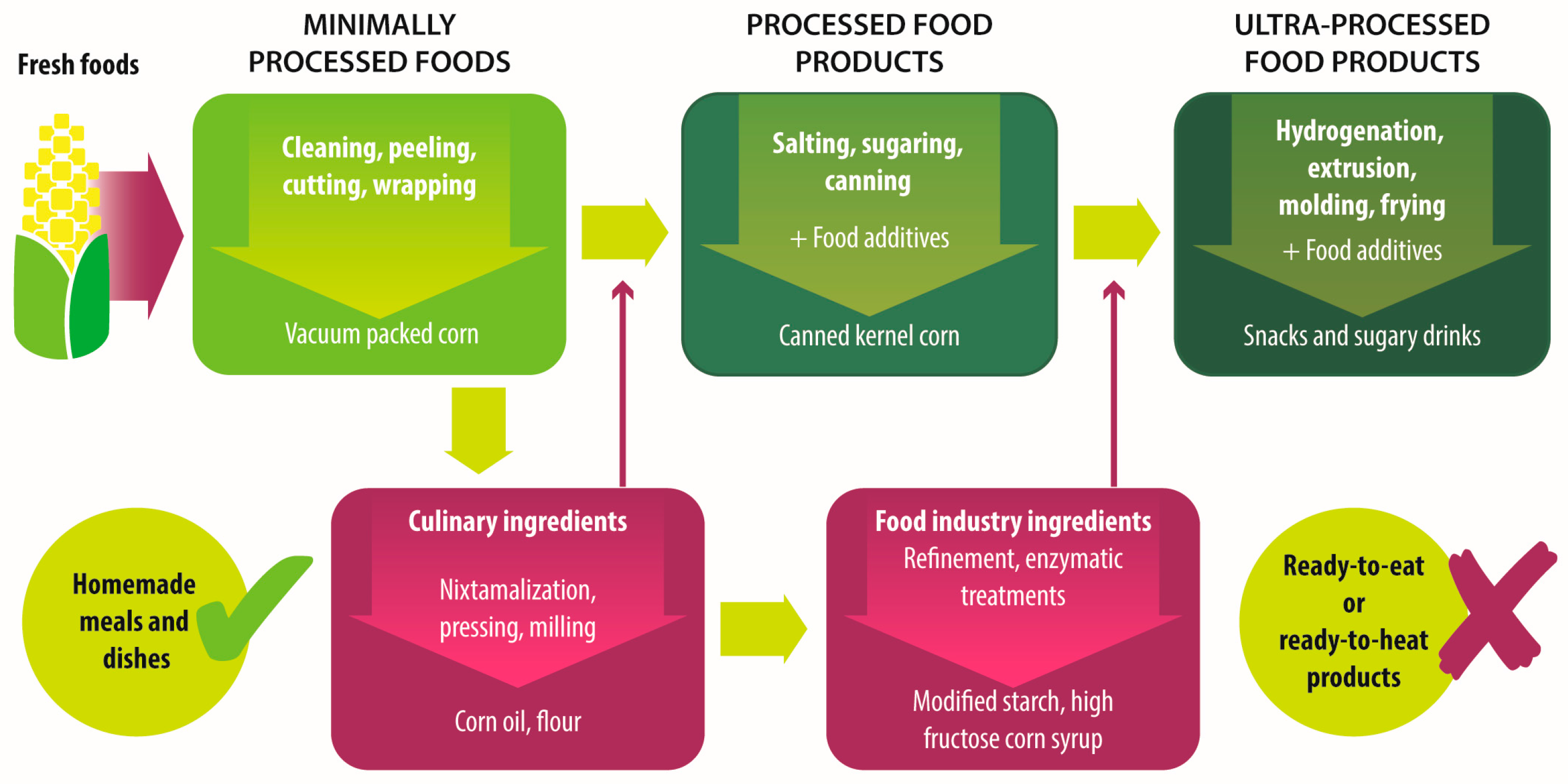
|
Group |
Definition |
Processing |
Examples |
|---|---|---|---|
|
Unprocessed foods |
Fresh foods directly obtained from plants or animals. |
No industrial processing. |
Fresh fruits, vegetables, meat, eggs, grains and legumes. |
|
Minimally processed foods |
Physical alteration of unprocessed foods. |
Peeling, cutting, drying, pasteurization, refrigeration, freezing, vacuum packing, simple wrapping. |
Chilled, frozen or dried fruits, vegetables, meat and poultry; pasteurized or powdered milk; vegetables or fruit juices without added sugar. |
|
Processed culinary ingredients |
Substances extracted from unprocessed or minimally processed foods used to prepare dishes and meals. |
Pressing, refining, grinding, milling. |
Salt, sugar, flour, vegetable oil, starches, butter, etc. |
|
Processed food industry ingredients |
Substances extracted from unprocessed or minimally processed foods used in the formulation of ultra-processed foods. |
Hydrogenation, hydrolysis, use of enzymes and additives. |
High fructose corn syrup, lactose, milk and soy proteins. |
|
Processed foods |
Products made by adding sugar, salt, oil, fats or other culinary ingredients, to minimally processed foods. |
Preservation or cooking methods, non-alcoholic fermentation. |
Bread, cheese, canned vegetables and legumes, fruits in syrup, salted nuts and seeds, smoked and salted meat. |
|
Ultra-processed foods |
Industrial formulations manufactured mainly from processed food industry ingredients. |
Frying, deep frying, curing, extrusion, molding, extensive use of additives, such as preservatives, colorants, flavorings, non-sugar sweeteners, emulsifiers, etc. |
Ready-to-heat, ready-to-eat or ready-to-drink products like carbonated drinks, sweet or savory snacks, breakfast cereals, fruit yoghurt, sausages, hams, instant soups, pre-prepared meals and dishes, infant formulas, baby food. |
4. Dietary Components Shape Gut Microbiota
5. Are Old Fashioned Diets Better?
|
Characteristic |
Old Fashioned Diet (Unprocessed or Minimally Processed Food-Based Diet) |
Ultra-Processed Products-Based Diet |
|---|---|---|
|
Fiber * |
↑ Dietary fiber from vegetables, whole grains and cereals. |
↓ Dietary fiber due to the refining process. |
|
Fat * |
Balance between saturated and unsaturated fats, depending on food selection. |
↑ Total fat and trans fat added or generated by the processes of baking and frying. |
|
Carbohydrates * |
↑ Complexes carbohydrates and natural resistant starch from whole grains and cereals. |
↑ Added sugars in sweets, confectionary and soft drinks. |
|
Protein * |
↑ Amount and quality of protein from fresh meat, eggs, fish and poultry. |
↓ Quantity of protein often accompanied by added fat. |
|
Micronutrients * |
↑ Quantity of vitamins and minerals if all food groups are included in a balanced way. |
↓ Concentration of vitamins and minerals due to the refining process if not fortified. |
|
Sodium * |
Sodium intake depends mainly on the added salt to foods. |
↑ Amounts of sodium. |
|
Additives * |
Free of additives. |
Extensive use of additives like emulsifiers, coloring, flavoring, and preservatives. |
|
Effect on ç: |
||
|
Gut microbiota ‡ |
Eubiosis with high abundance of butyrate producer bacteria. |
Dysbiosis marked by Bacteroides and gram-negative Proteobacteria. |
|
Bacterial Metabolites γ |
↑ Production of butyrate |
↑ Production of acetate and other short chain fatty acids. |
|
Immune response § |
Anti-inflammatory response. |
Pro-inflammatory response. |
|
Epithelia integrity § |
Thigh junction’s integrity due to the production of butyrate. |
Altered intestinal permeability due to dysbiosis or emulsifiers’ effect. |
|
Susceptibility to T1D or CD ¶ |
Reduced susceptibility. |
Increased susceptibility. |
↑: higher; ↓: lower; T1D: type 1 diabetes; CD: celiac disease; * [15][16][20][21][22][23][24][25][26][27][28][29][30][38]; ç Comparing high in fiber and resistant starch vs. high in fat, sugars and emmulsifiers diets. ‡ [11][12][13][14][33][36][39][40][41][42][43][44][45][46][47][48][49][50][51][52][53][54]; γ [17][39][41][42][50]; § [14][17][34][39][55][56][57][58]; ¶ [11][17][31][55].
This entry is adapted from the peer-reviewed paper 10.3390/foods6110100
References
- American Diabetes Association. Classification and Diagnosis of Diabetes. Diabetes Care 2017, 40 (Suppl. 1), S11–S24.
- Ludvigsson, J.F.; Leffler, D.A.; Bai, J.C.; Biagi, F.; Fasano, A.; Green, P.H.; Hadjivassiliou, M.; Kaukinen, K.; Kelly, C.P.; Leonard, J.N.; et al. The Oslo definitions for coeliac disease and related terms. Gut 2013, 62, 43–52.
- Pettitt, D.J.; Talton, J.; Dabelea, D.; Divers, J.; Imperatore, G.; Lawrence, J.M.; Liese, A.D.; Linder, B.; Mayer-Davis, E.J.; Pihoker, C.; et al. Prevalence of diabetes in U.S. youth in 2009: The SEARCH for diabetes in youth study. Diabetes Care 2014, 37, 402–408.
- Ludvigsson, J.F.; Rubio-Tapia, A.; van Dyke, C.T.; Melton, L.J.; Zinsmeister, A.R.; Lahr, B.D.; Murray, J.A. Increasing incidence of celiac disease in a North American population. Am. J. Gastroenterol. 2013, 108, 818–824.
- Namatovu, F.; Sandström, O.; Olsson, C.; Lindkvist, M.; Ivarsson, A. Celiac disease risk varies between birth cohorts, generating hypotheses about causality: Evidence from 36 years of population-based follow-up. BMC Gastroenterol. 2014, 14, 59–66.
- Fazeli Farsani, S.; Souverein, P.C.; van der Vorst, M.M.; Knibbe, C.A.; Herings, R.M.; de Boer, A.; Mantel-Teeuwisse, A.K. Increasing trends in the incidence and prevalence rates of type 1 diabetes among children and adolescents in the Netherlands. Pediatr. Diabetes 2016, 17, 44–52.
- Hu, Y.; Wong, F.S.; Wen, L. Antibiotics, gut microbiota, environment in early life and type 1 diabetes. Pharmacol. Res. 2017, 119, 219–226.
- Verdu, E.F.; Galipeau, H.J.; Jabri, B. Novel players in coeliac disease pathogenesis: Role of the gut microbiota. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 497–506.
- Knip, M.; Siljander, H. The role of the intestinal microbiota in type 1 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 154–167.
- Gensollen, T.; Iyer, S.S.; Kasper, D.L.; Blumberg, R.S. How colonization by microbiota in early life shapes the immune system. Science 2016, 352, 539–544.
- Endesfelder, D.; Engel, M.; Davis-Richardson, A.G.; Ardissone, A.N.; Achenbach, P.; Hummel, S.; Winkler, C.; Atkinson, M.; Schatz, D.; Triplett, E.; et al. Towards a functional hypothesis relating anti-islet cell autoimmunity to the dietary impact on microbial communities and butyrate production. Microbiome 2016, 4, 17–28.
- Wu, G.D.; Chen, J.; Hoffmann, C.; Bittinger, K.; Chen, Y.Y.; Keilbaugh, S.A.; Bewtra, M.; Knights, D.; Walters, W.A.; Knight, R.; et al. Linking long-term dietary patterns with gut microbial enterotypes. Science 2011, 334, 105–108.
- David, L.A.; Maurice, C.F.; Carmody, R.N.; Gootenberg, D.B.; Button, J.E.; Wolfe, B.E.; Ling, A.V.; Devlin, A.S.; Varma, Y.; Fischbach, M.; et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature 2014, 505, 559–563.
- Davis-Richardson, A.G.; Triplett, E.W. A model for the role of gut bacteria in the development of autoimmunity for type 1 diabetes. Diabetologia 2015, 58, 1386–1393.
- Monteiro, C.A.; Levy, R.B.; Claro, R.M.; Castro, I.R.; Cannon, G. A new classification of foods based on the extent and purpose of their processing. Cad. Saude Publica 2010, 26, 2039–2049.
- Monteiro, C.A.; Moubarac, J.C.; Levy, R.B.; Canella, D.S.; Louzada, M.L.D.C.; Cannon, G. Household availability of ultra-processed foods and obesity in nineteen European countries. Public Health Nutr. 2017, in press.
- Mejía-León, M.E.; Calderón de la Barca, A.M. Diet, Microbiota and Immune System in Type 1 Diabetes Development and Evolution. Nutrients 2015, 7, 9171–9184.
- Mejía-León, M.E.; Petrosino, J.F.; Ajami, N.J.; Domínguez-Bello, M.G.; Calderón de la Barca, A.M. Fecal microbiota imbalance in Mexican children with type 1 diabetes. Sci. Rep. 2014, 4, 3814.
- Ríos-Covián, D.; Ruas-Madiedo, P.; Margolles, A.; Gueimonde, M.; de Los Reyes-Gavilán, C.G.; Salazar, N. Intestinal Short Chain Fatty Acids and their Link with Diet and Human Health. Front. Microbiol. 2016, 7, 185.
- Moubarac, J.C.; Batal, M.; Martins, A.P.; Claro, R.; Levy, R.B.; Cannon, G.; Monteiro, C. Processed and ultra-processed food products: Consumption trends in Canada from 1938 to 2011. Can. J. Diet. Pract. Res. 2014, 75, 15–21.
- Djupegot, I.L.; Nenseth, C.B.; Bere, E.; Bjørnarå, H.B.T.; Helland, S.H.; Øverby, N.C.; Torstveit, M.K.; Stea, T.H. The association between time scarcity, sociodemographic correlates and consumption of ultra-processed foods among parents in Norway: A cross-sectional study. BMC Public Health 2017, 17, 447.
- Pan American Health Organization; World Health Organization regional office for the Americas. Ultra-Processed Food and Drink Products in Latin America: Trends, Impact on Obesity, Policy Implications; PAHO: Washington, DC, USA, 2015.
- Fardet, A. Minimally processed foods are more satiating and less hyperglycemic than ultra-processed foods: A preliminary study with 98 ready-to-eat foods. Food Funct. 2016, 7, 2338–2346.
- Moubarac, J.C.; Batal, M.; Louzada, M.L.; Martinez Steele, E.; Monteiro, C.A. Consumption of ultra-processed foods predicts diet quality in Canada. Appetite 2017, 108, 512–520.
- Martins, A.P.; Levy, R.B.; Claro, R.M.; Moubarac, J.C.; Monteiro, C.A. Increased contribution of ultra-processed food products in the Brazilian diet (1987–2009). Rev. Saude Publica 2013, 47, 656–665.
- Mendonça, R.D.; Lopes, A.C.; Pimenta, A.M.; Gea, A.; Martinez-Gonzalez, M.A.; Bes-Rastrollo, M. Ultra-Processed Food Consumption and the Incidence of Hypertension in a Mediterranean Cohort: The Seguimiento Universidad de Navarra Project. Am. J. Hypertens. 2017, 30, 358–366.
- Giménez, A.; Saldamando, L.; Curutchet, M.R.; Ares, G. Package design and nutritional profile of foods targeted at children in supermarkets in Montevideo, Uruguay. Cad. Saude Publica 2017, 33, e00032116.
- Karnopp, E.V.; Vaz, J.D.; Schafer, A.A.; Muniz, L.C.; Souza, R.L.; Santos, I.D.; Gigante, D.P.; Assunção, M.C. Food consumption of children younger than 6 years according to the degree of food processing. J. Pediatr. (Rio J.) 2017, 93, 70–78.
- Tavares, L.F.; Fonseca, S.C.; Garcia Rosa, M.L.; Yokoo, E.M. Relationship between ultra-processed foods and metabolic syndrome in adolescents from a Brazilian Family Doctor Program. Public Health Nutr. 2012, 15, 82–87.
- Rauber, F.; Campagnolo, P.D.; Hoffman, D.J.; Vitolo, M.R. Consumption of ultra-processed food products and its effects on children’s lipid profiles: A longitudinal study. Nutr. Metab. Cardiovasc. Dis. 2015, 25, 116–122.
- Wilkin, T.J. The convergence of type 1 and type 2 diabetes in childhood: The accelerator hypothesis. Pediatr. Diabetes 2012, 13, 334–339.
- Yatsunenko, T.; Rey, F.E.; Manary, M.J.; Trehan, I.; Dominguez-Bello, M.G.; Contreras, M.; Magris, M.; Hidalgo, G.; Baldassano, R.N.; Anokhin, A.P.; et al. Human gut microbiome viewed across age and geography. Nature 2012, 486, 222–227.
- De Filippo, C.; Cavalieri, D.; Di Paola, M.; Ramazzotti, M.; Poullet, J.B.; Massart, S.; Collini, S.; Pieraccini, G.; Lionetti, P. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc. Natl. Acad. Sci. USA 2010, 107, 14691–14696.
- Chassaing, B.; Van de Wiele, T.; Gewirtz, A. Dietary Emulsifiers Directly Impact the Human Gut Microbiota Increasing Its Pro-inflammatory Potential and Ability to Induce Intestinal Inflammation. Inflamm. Bowel Dis. 2017, 23, S5.
- Liu, Z.; Roy, N.C.; Guo, Y.; Jia, H.; Ryan, L.; Samuelsson, L.; Thomas, A.; Plowman, J.; Clerens, S.; Day, L.; et al. Human Breast Milk and Infant Formulas Differentially Modify the Intestinal Microbiota in Human Infants and Host Physiology in Rats. J. Nutr. 2016, 146, 191–199.
- Kashtanova, D.A.; Popenko, A.S.; Tkacheva, O.N.; Tyakht, A.B.; Alexeev, D.G.; Boytsov, S.A. Association between the gut microbiota and diet: Fetal life, early childhood, and further life. Nutrition 2016, 32, 620–627.
- Wopereis, H.; Oozeer, R.; Knipping, K.; Belzer, C.; Knol, J. The first thousand days—Intestinal microbiology of early life: Establishing a symbiosis. Pediatr. Allergy Immunol. 2014, 25, 428–438.
- Martínez Steele, E.; Baraldi, L.G.; Louzada, M.L.; Moubarac, J.C.; Mozaffarian, D.; Monteiro, C.A. Ultra-processed foods and added sugars in the US diet: Evidence from a nationally representative cross-sectional study. BMJ Open 2016, 6, e009892.
- Jiminez, J.A.; Uwiera, T.C.; Abbott, D.W.; Uwiera, R.R.; Inglis, G.D. Impacts of resistant starch and wheat bran consumption on enteric inflammation in relation to colonic bacterial community structures and short-chain fatty acid concentrations in mice. Gut Pathog. 2016, 8, 67.
- Upadhyaya, B.; McCormack, L.; Fardin-Kia, A.R.; Juenemann, R.; Nichenametla, S.; Clapper, J.; Specker, B.; Dey, M. Impact of dietary resistant starch type 4 on human gut microbiota and immunometabolic functions. Sci. Rep. 2016, 6, 28797.
- Hald, S.; Schioldan, A.G.; Moore, M.E.; Dige, A.; Lærke, H.N.; Agnholt, J.; Bach Knudsen, K.E.; Hermansen, K.; Marco, M.L.; Gregersen, S.; et al. Effects of Arabinoxylan and Resistant Starch on Intestinal Microbiota and Short-Chain Fatty Acids in Subjects with Metabolic Syndrome: A Randomised Crossover Study. PLoS ONE 2016, 11, e0159223.
- Nakayama, J.; Yamamoto, A.; Palermo-Conde, L.A.; Higashi, K.; Sonomoto, K.; Tan, J.; Lee, Y.K. Impact of Westernized Diet on Gut Microbiota in Children on Leyte Island. Front. Microbiol. 2017, 8, 197.
- Byerley, L.O.; Samuelson, D.; Blanchard, E.; Luo, M.; Lorenzen, B.N.; Banks, S.; Ponder, M.A.; Welsh, D.A.; Taylor, C.M. Changes in the gut microbial communities following addition of walnuts to the diet. J. Nutr. Biochem. 2017, 48, 94–102.
- Robertson, R.C.; Seira Oriach, C.; Murphy, K.; Moloney, G.M.; Cryan, J.F.; Dinan, T.G.; Paul Ross, R.; Stanton, C. Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav. Immun. 2017, 59, 21–37.
- Noriega, B.S.; Sanchez-Gonzalez, M.A.; Salyakina, D.; Coffman, J. Understanding the Impact of Omega-3 Rich Diet on the Gut Microbiota. Case Rep. Med. 2016, 2016, 3089303.
- Bi, X.; Li, F.; Liu, S.; Jin, Y.; Zhang, X.; Yang, T.; Dai, Y.; Li, X.; Zhao, A.Z. ω-3 polyunsaturated fatty acids ameliorate type 1 diabetes and autoimmunity. J. Clin. Investig. 2017, 127, 1757–1771.
- Bergamo, P.; Palmieri, G.; Cocca, E.; Ferrandino, I.; Gogliettino, M.; Monaco, A.; Maurano, F.; Rossi, M. Adaptive response activated by dietary cis9, trans11 conjugated linoleic acid prevents distinct signs of gliadin-induced enteropathy in mice. Eur. J. Nutr. 2016, 55, 729–740.
- Onishi, J.C.; Campbell, S.; Moreau, M.; Patel, F.; Brooks, A.I.; Zhou, Y.X.; Häggblom, M.M.; Storch, J. Bacterial communities in the small intestine respond differently to those in the caecum and colon in mice fed low- and high-fat diets. Microbiology 2017, 163, 1189–1197.
- Agus, A.; Denizot, J.; Thévenot, J.; Martinez-Medina, M.; Massier, S.; Sauvanet, P.; Bernalier-Donadille, A.; Denis, S.; Hofman, P.; Bonnet, R.; et al. Western diet induces a shift in microbiota composition enhancing susceptibility to Adherent-Invasive E. coli infection and intestinal inflammation. Sci. Rep. 2016, 6, 19032.
- Shankar, V.; Gouda, M.; Moncivaiz, J.; Gordon, A.; Reo, N.V.; Hussein, L.; Paliy, O. Differences in Gut Metabolites and Microbial Composition and Functions between Egyptian and U.S. Children Are Consistent with Their Diets. mSystems 2017, 2, e00169-16.
- Zhu, Y.; Lin, X.; Zhao, F.; Shi, X.; Li, H.; Li, Y.; Zhu, W.; Xu, X.; Li, C.; Zhou, G. Meat, dairy and plant proteins alter bacterial composition of rat gut bacteria. Sci. Rep. 2015, 5, 15220.
- Avila-Nava, A.; Noriega, L.G.; Tovar, A.R.; Granados, O.; Perez-Cruz, C.; Pedraza-Chaverri, J.; Torres, N. Food combination based on a pre-hispanic Mexican diet decreases metabolic and cognitive abnormalities and gut microbiota dysbiosis caused by a sucrose-enriched high-fat diet in rats. Mol. Nutr. Food Res. 2017, 61, 1501023.
- Gutiérrez-Díaz, I.; Fernández-Navarro, T.; Sánchez, B.; Margolles, A.; González, S. Mediterranean diet and faecal microbiota: A transversal study. Food Funct. 2016, 7, 2347–2356.
- Glick-Bauer, M.; Yeh, M.C. The health advantage of a vegan diet: Exploring the gut microbiota connection. Nutrients 2014, 6, 4822–4838.
- Lerner, A.; Matthias, T. Changes in intestinal tight junction permeability associated with industrial food additives explain the rising incidence of autoimmune disease. Autoimmun. Rev. 2015, 14, 479–489.
- Csáki, K.F. Synthetic surfactant food additives can cause intestinal barrier dysfunction. Med. Hypotheses 2011, 76, 676–681.
- Chassaing, B.; Koren, O.; Goodrich, J.K.; Poole, A.C.; Srinivasan, S.; Ley, R.E.; Gewirtz, A.T. Dietary emulsifiers impact the mouse gut microbiota promoting colitis and metabolic syndrome. Nature 2015, 519, 92–96.
- Swidsinski, A.; Ung, V.; Sydora, B.C.; Loening-Baucke, V.; Doerffel, Y.; Verstraelen, H.; Fedorak, R.N. Bacterial overgrowth and inflammation of small intestine after carboxymethylcellulose ingestion in genetically susceptible mice. Inflamm. Bowel Dis. 2009, 15, 359–364.
