Point-of-care testing (POCT) technology has exhibited an outstanding capability for the detection of several disease biomarkers owing to the fact that such techniques are fast, easy to perform, efficient, and low cost. The lateral flow immunoassay (LFIA) is one such strategy for POCT. LFIA is a well-established platform and a potent assay for fast and inexpensive testing, as this technology is instrumentation independent and allows the visualisation of test results by the naked eye. In order to enhance the sensitivity and specificity of the LFIAs, as well as to allow the quantitation of results, fluorescence immunochromatographic assays have been developed by utilising fluorescent reporters. Fluorescence immunochromatographic assays have advantages over conventional approaches in regard to sensitivity as it produces a higher intensity band on the test and control lines. One such promising fluorescent reporter is quantum dots (QDs). QDs are tiny semiconducting nanocrystals with diameters ranging from 2 to 10 nanometers. QDs have unique electronic characteristics that are intermediate between those of bulk semiconductors and discrete molecules, which is due in part to their high surface-to-volume ratios. The most visible result is fluorescence, in which the nanocrystals emit distinct colours determined by particle size.
- quantum dot
- lateral flow immunoassay
- infectious diseases
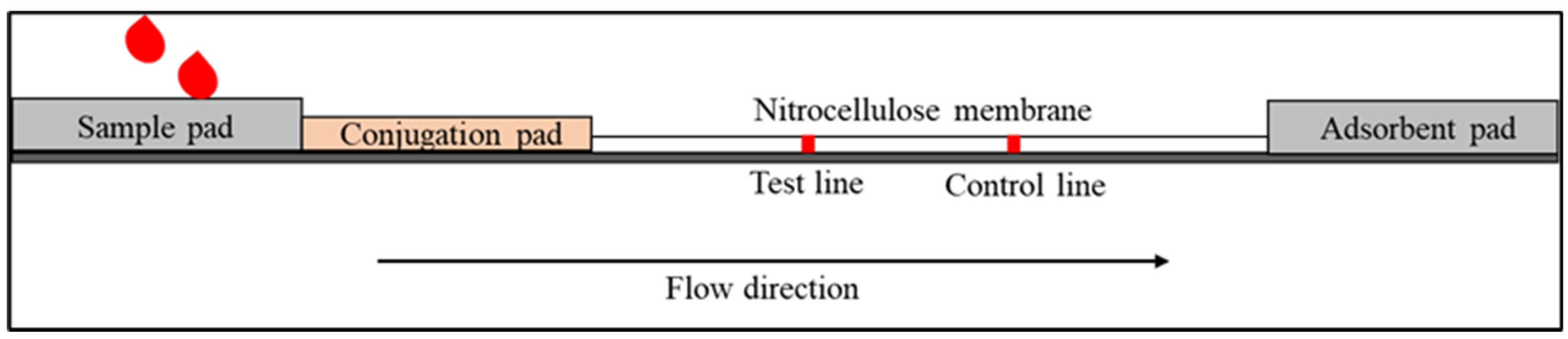
1. Lateral Flow Immunoassay
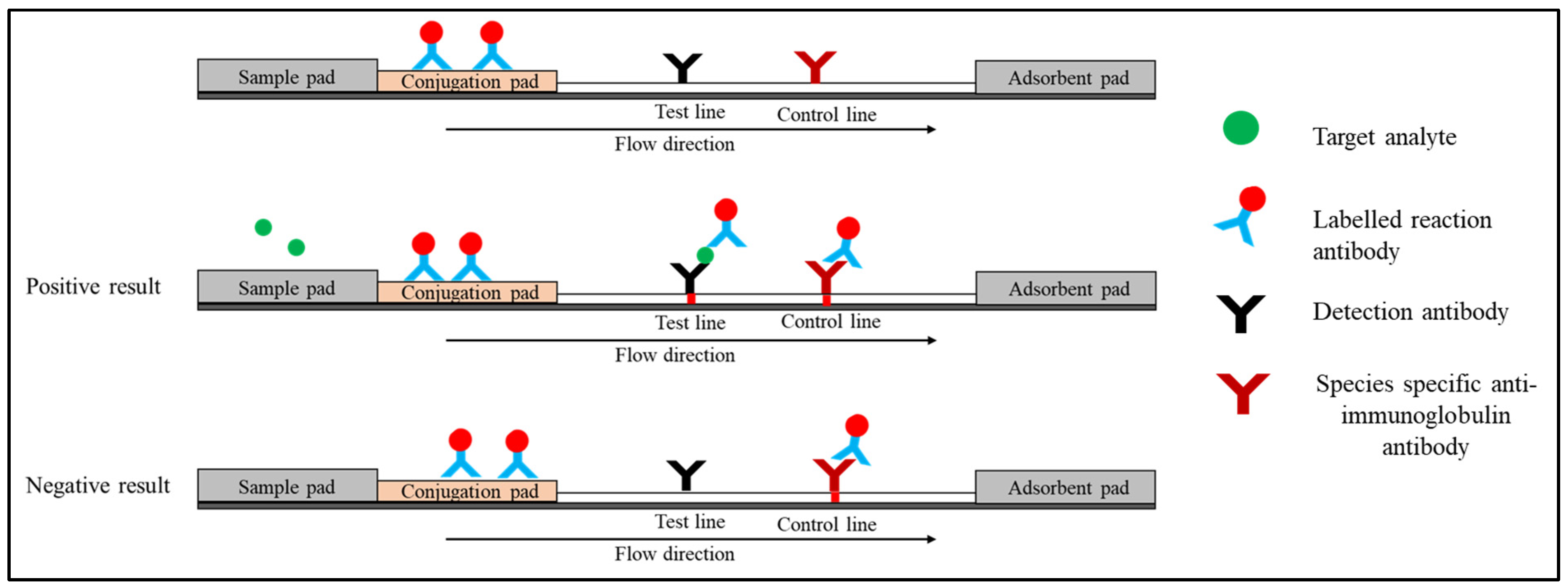
1.1. Sandwich Immunoassay
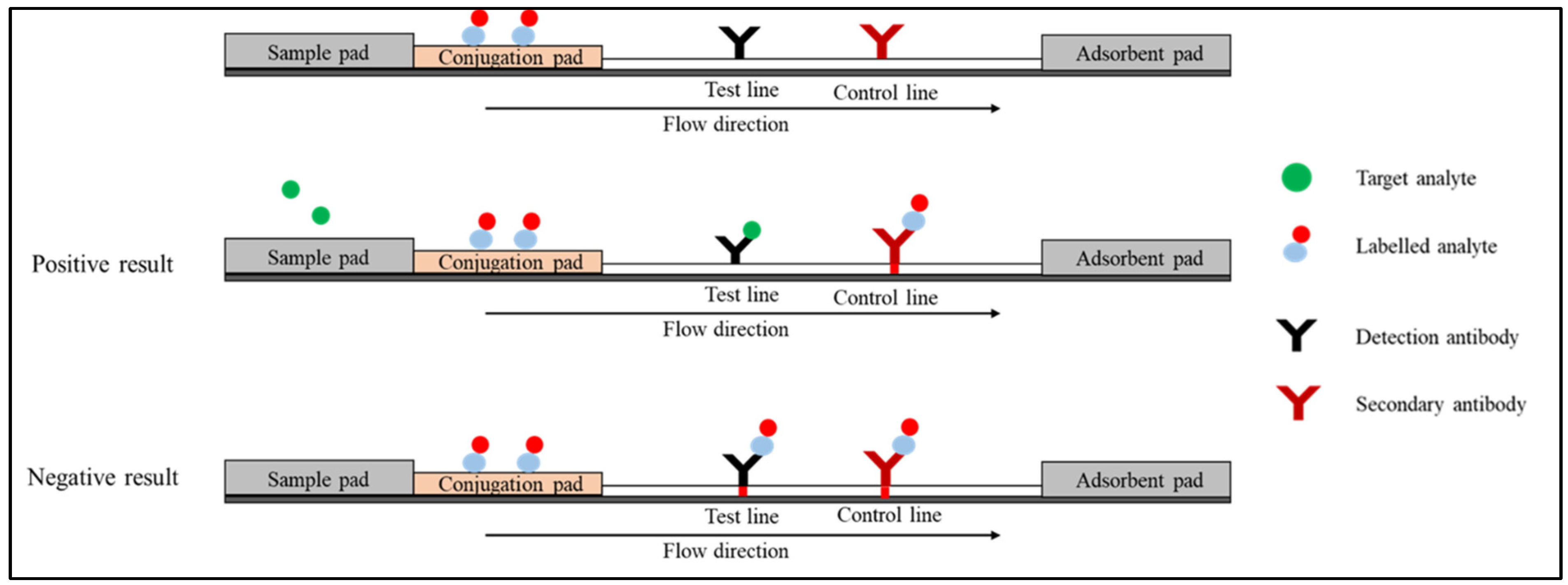
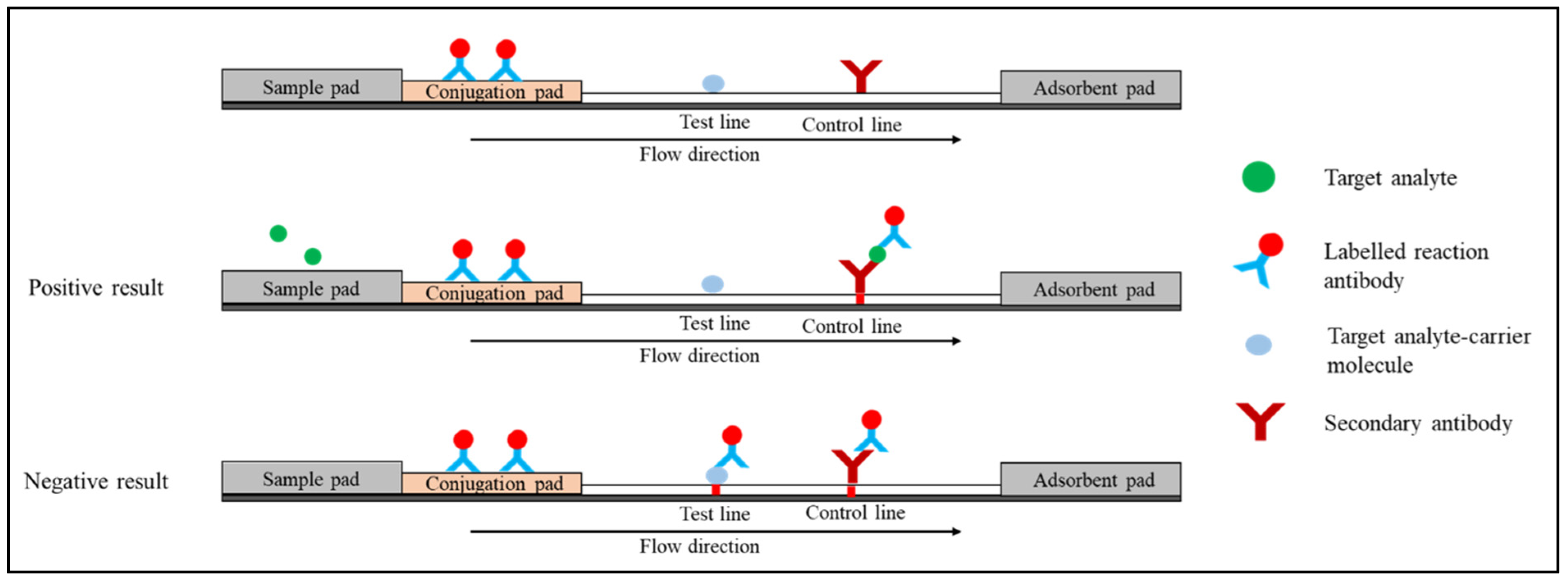
1.2. Competitive Immunoassay (or Inhibition Immunoassay)
2. Quantum Dot-Based Lateral Flow Immunoassay
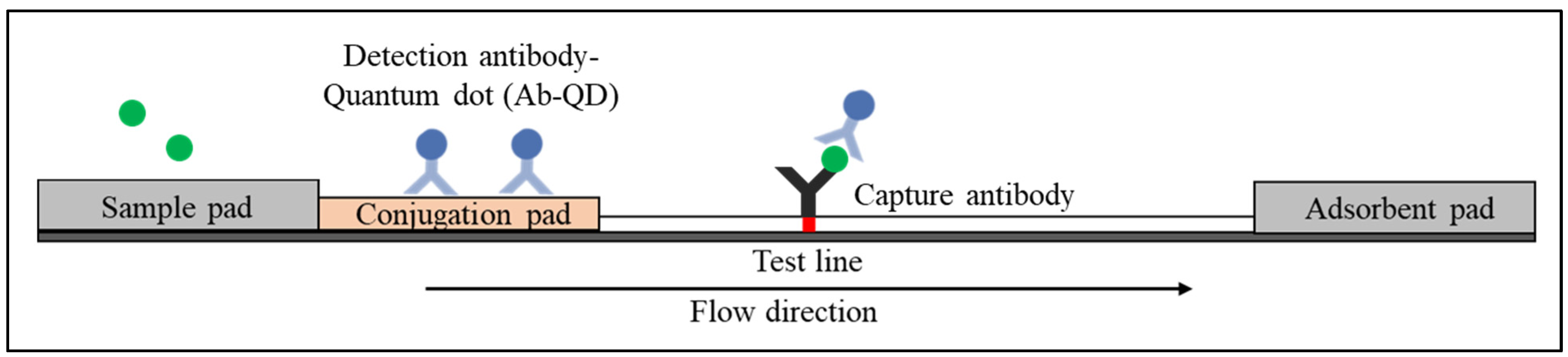
2.1. Types of Quantum Dots
2.2. Strategies for Conjugating Quantum Dot with Antibodies
2.3. Performance of Quantum Dot-Based Lateral Flow Immunoassay
Syphilis is a sexually transmitted infection (STI) that causes serious health problems worldwide. In 2010, Yang et al. [21] developed a QD-LFIA to be used for the screening of syphilis. The QD-LFIA was designed to detect anti-TP47 polyclonal antibodies by visualising the emission of CdTe under a portable ultraviolet lamp. Thioglycolic acid (TGA) was used to link the QDs with Staphylococcal Protein A (SPA). In the presence of anti-TP47 polyclonal antibodies, the QD-labelled SPA will form a complex with the antibodies. Then, the anti-TP47 antibodies will bind to the TP47 antigen immobilised on the test line, producing a signal that can be visualised under UV light. The assay is suitable for rapid indirect screening of syphilis as the turnaround time is only 10 min. With regard to the limit of detection, the QD-LFIA could detect as low as 2 ng/mL, which was tenfold higher than that of the AuNPs–based method.
Tuberculosis, an infectious disease caused by the Mycobacterium tuberculosis, is a global public health problem and among the top 10 leading causes of death worldwide, particularly in low- and middle-income countries [22]. In an effort to prevent the spread of the disease, a QD-LFIA device based on a double-antibody sandwich format labelled with core–shell quantum dots (CdSe/ZnS) coupled with streptavidin was developed for the detection of M. tuberculosis fprA proteins [23]. It reported that the device was able to detect fprA proteins in liquid samples at the lowest dilution of 12.5 pg/µL. The sensitivity of LFIA improved as compared to other immunochromatographic tests following the use of QDs labelled antibodies.
This entry is adapted from the peer-reviewed paper 10.3390/diagnostics12092158
References
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120.
- Singh, J.; Sharma, S.; Nara, S. Evaluation of Gold Nanoparticle Based Lateral Flow Assays for Diagnosis of Enterobacteriaceae Members in Food and Water. Food Chem. 2015, 170, 470–483.
- Mao, X.; Wang, W.; Du, T.-E. Rapid Quantitative Immunochromatographic Strip for Multiple Proteins Test. Sensors Actuators B Chem. 2013, 186, 315–320.
- Biagini, R.E.; Sammons, D.L.; Smith, J.P.; MacKenzie, B.A.; Striley, C.A.F.; Snawder, J.E.; Robertson, S.A.; Quinn, C.P. Rapid, Sensitive, and Specific Lateral-Flow Immunochromatographic Device To Measure Anti-Anthrax Protective Antigen Immunoglobulin G in Serum and Whole Blood. Clin. Vaccine Immunol. 2006, 13, 541–546.
- Gupta, Y.; Ghrera, A.S. Recent Advances in Gold Nanoparticle-Based Lateral Flow Immunoassay for the Detection of Bacterial Infection. Arch. Microbiol. 2021, 203, 3767–3784.
- Vemulapati, S.; Rey, E.; O’Dell, D.; Mehta, S.; Erickson, D. A Quantitative Point-of-Need Assay for the Assessment of Vitamin D3 Deficiency. Sci. Rep. 2017, 7, 14142.
- Bahadır, E.B.; Sezgintürk, M.K. Lateral Flow Assays: Principles, Designs and Labels. TrAC Trends Anal. Chem. 2016, 82, 286–306.
- Mosley, G.L.; Pereira, D.Y.; Han, Y.; Lee, S.Y.; Wu, C.M.; Wu, B.M.; Kamei, D.T. Improved Lateral-Flow Immunoassays for Chlamydia and Immunoglobulin M by Sequential Rehydration of Two-Phase System Components within a Paper-Based Diagnostic. Microchim. Acta 2017, 184, 4055–4064.
- Lin, L.; Guo, J.; Liu, H.; Jiang, X. Rapid Detection of Hepatitis B Virus in Blood Samples Using a Combination of Polymerase Spiral Reaction With Nanoparticles Lateral-Flow Biosensor. Front. Mol. Biosci. 2021, 7, 578892.
- Kim, C.; Yoo, Y.K.; Han, S.I.; Lee, J.; Lee, D.; Lee, K.; Hwang, K.S.; Lee, K.H.; Chung, S.; Lee, J.H. Battery Operated Preconcentration-Assisted Lateral Flow Assay. Lab Chip 2017, 17, 2451–2458.
- Gwyn, S.; Mitchell, A.; Dean, D.; Mkocha, H.; Handali, S.; Martin, D.L. Lateral Flow-Based Antibody Testing for Chlamydia Trachomatis. J. Immunol. Methods 2016, 435, 27–31.
- Sajid, M.; Kawde, A.-N.; Daud, M. Designs, Formats and Applications of Lateral Flow Assay: A Literature Review. J. Saudi Chem. Soc. 2015, 19, 689–705.
- Alhussien, M.N.; Dang, A.K. Sensitive and Rapid Lateral-Flow Assay for Early Detection of Subclinical Mammary Infection in Dairy Cows. Sci. Rep. 2020, 10, 11161.
- Hwang, E.; Hwang, H.M.; Shin, Y.; Yoon, Y.; Lee, H.; Yang, J.; Bak, S.; Lee, H. Chemically Modulated Graphene Quantum Dot for Tuning the Photoluminescence as Novel Sensory Probe. Sci. Rep. 2016, 6, 39448.
- Schroeder, K.L.; Goreham, R.V.; Nann, T. Graphene Quantum Dots for Theranostics and Bioimaging. Pharm. Res. 2016, 33, 2337–2357.
- Mansur, H.S.; Mansur, A.A.P. CdSe Quantum Dots Stabilized by Carboxylic-Functionalized PVA: Synthesis and UV–Vis Spectroscopy Characterization. Mater. Chem. Phys. 2011, 125, 709–717.
- Chen, L.; Zhang, C.; Du, Z.; Li, H.; Zhang, L.; Zou, W. Fabrication of Carboxyl Group-Functionalized Carbon Quantum Dots and Its Transparent and Luminescent Epoxy Matrix Nanocomposites for White LED Encapsulation. Macromol. Mater. Eng. 2015, 300, 1232–1237.
- Wang, S.; Liu, Y.; Jiao, S.; Zhao, Y.; Guo, Y.; Wang, M.; Zhu, G. Quantum-Dot-Based Lateral Flow Immunoassay for Detection of Neonicotinoid Residues in Tea Leaves. J. Agric. Food Chem. 2017, 65, 10107–10114.
- Li, M.; Ma, M.; Hua, X.; Shi, H.; Wang, Q.; Wang, M. Quantum Dots-Based Fluoroimmunoassay for the Simultaneous Detection of Clothianidin and Thiacloprid in Environmental and Agricultural Samples. RSC Adv. 2015, 5, 3039–3044.
- Foubert, A.; Beloglazova, N.V.; Rajkovic, A.; Sas, B.; Madder, A.; Goryacheva, I.Y.; De Saeger, S. Bioconjugation of Quantum Dots: Review & Impact on Future Application. TrAC Trends Anal. Chem. 2016, 83, 31–48.
- Yang, H.; Li, D.; He, R.; Guo, Q.; Wang, K.; Zhang, X.; Huang, P.; Cui, D. A Novel Quantum Dots–Based Point of Care Test for Syphilis. Nanoscale Res. Lett. 2010, 5, 875–881.
- López-López, J.P.; Posada-Martínez, E.L.; Saldarriaga, C.; Wyss, F.; Ponte-Negretti, C.I.; Alexander, B.; Miranda-Arboleda, A.F.; Martínez-Sellés, M.; Baranchuk, A. Tuberculosis and the Heart. J. Am. Heart Assoc. 2021, 10, e019435.
- Cimaglia, F.; Aliverti, A.; Chiesa, M.; Poltronieri, P.; De Lorenzis, E.; Santino, A.; Sechi, L.A. Quantum Dots Nanoparticle-Based Lateral Flow Assay for Rapid Detection of Mycobacterium Species Using Anti-FprA Antibodies. Nanotechnol. Dev. 2012, 2, 5.
