Sugarcane is a lignocellulosic crop and the juice extracted from its stalks provides the raw material for 86% of sugar production. Globally, sugarcane processing to obtain sugar and/or ethanol generates more than 279 million tons of solid and liquid waste annually, as well as by-products; namely, straws, bagasse, press mud, wastewater, ash from bagasse incineration, vinasse from ethanol distillation, and molasses. If not properly managed, this waste will pose risks to both environmental factors and human health. Valorization of waste has gained momentum, having an important contribution to the fulfillment of policies and objectives related to sustainable development and circular bioeconomy. Various technologies are well-established and implemented for the valorization of waste and by-products from sugarcane processing, while other innovative technologies are still in the research and development stage, with encouraging prospects.
- biomass
- pretreatment
- cogeneration
- bioethanol
- bio-hydrogen
- compost
- fertilizer
- wastewater
1. Introduction
2. Waste and By-Products Generated in Sugarcane Processing
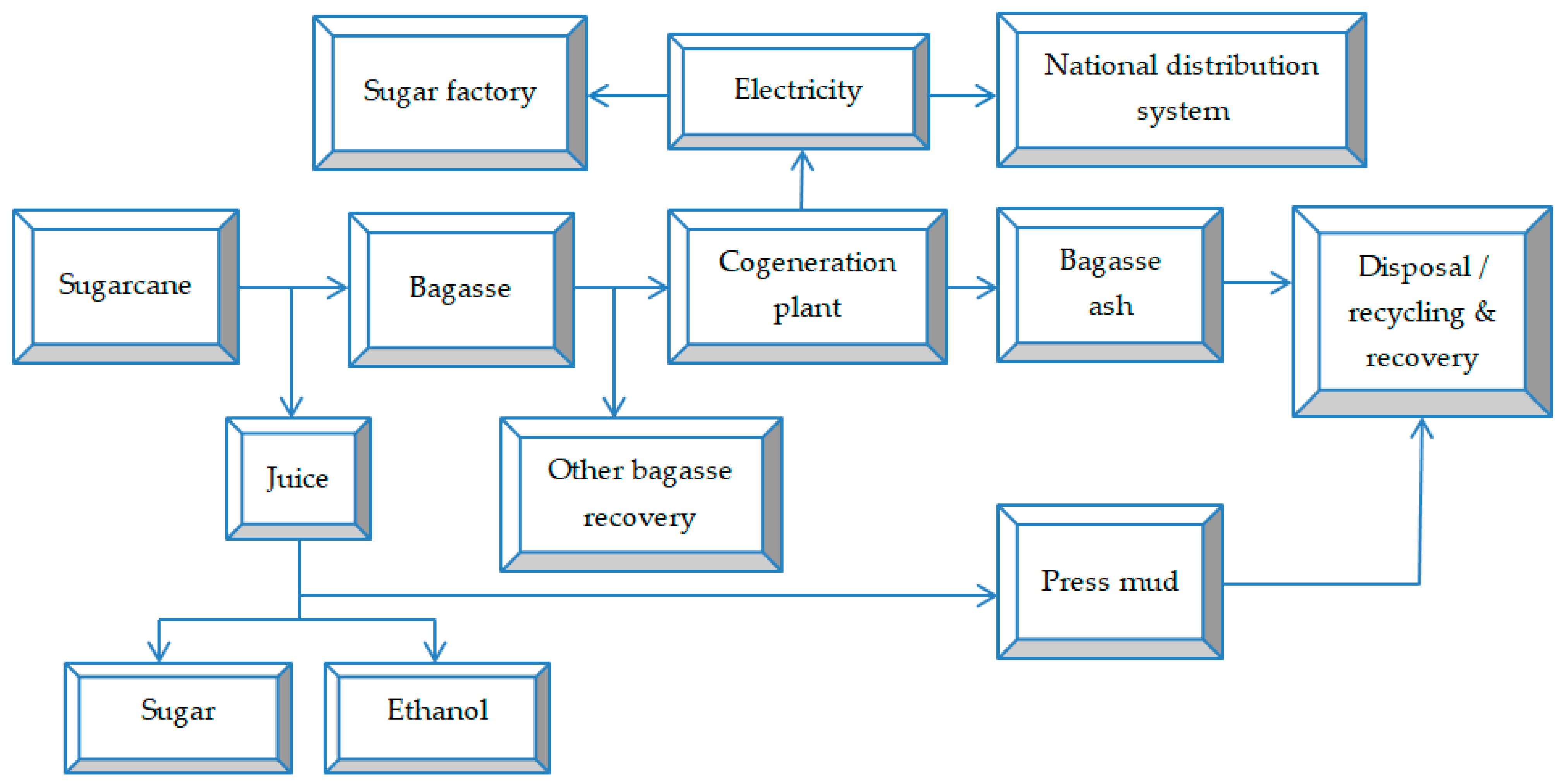
2.1. Waste and By-Product Generation Flow in Sugarcane Processing Factories
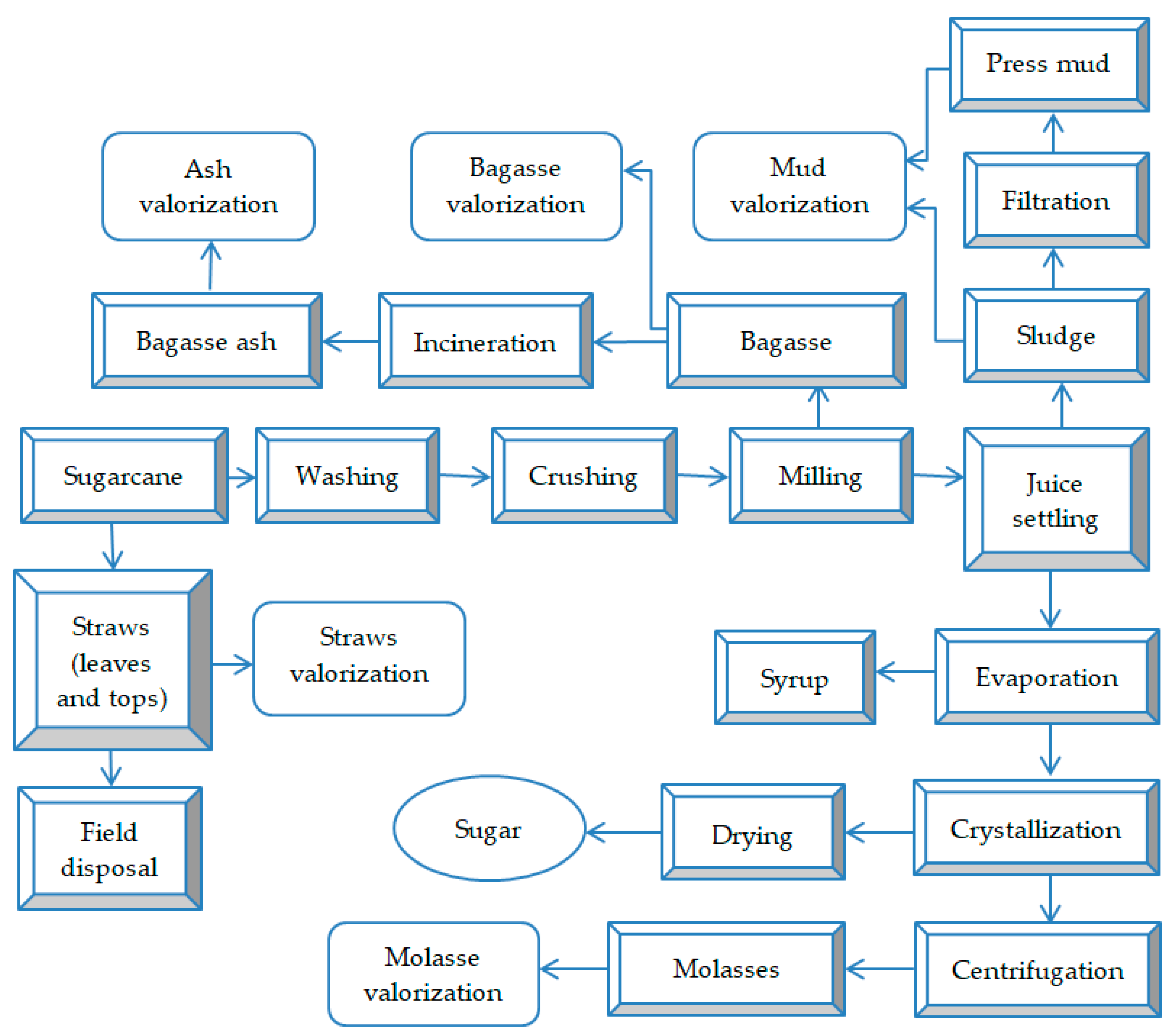
2.2. Sugarcane Leaves and Tops (Straws)
2.3. Sugarcane Bagasse
2.3.1. Characterization of Sugarcane Bagasse
2.3.2. Uncontrolled Disposal of Sugarcane Bagasse
2.3.3. Valorization of Sugarcane Bagasse
Incineration of Sugarcane Bagasse for Energy Recovery
2.4. Residual Ash from Bagasse Incineration
2.4.1. Characterization of Sugarcane Bagasse Ash
2.4.2. Valorization of Sugarcane Bagasse Ash
2.5. Vinasse from Ethanol Distillation
2.5.1. Characterization of Sugarcane Vinasse
2.5.2. Valorization of Sugarcane Vinasse
2.6. Press Mud (Cake)
2.6.1. Characterization of Sugarcane Press Mud
2.6.2. Valorization of Sugarcane Press Mud
2.7. Wastewater from Sugarcane Processing
2.7.1. Characterization of Wastewater from Sugarcane Processing
2.7.2. Valorization of Wastewater from Sugarcane Processing
2.8. Sugarcane Molasses
2.8.1. Characterization of Sugarcane Molasses
2.8.2. Valorization of Sugarcane Molasses
This entry is adapted from the peer-reviewed paper 10.3390/su141711089
References
- Mohamad, N.; Lakhiar, M.T.; Samad, A.A.A.; Mydin, A.O.; Jhatial, A.A.; Sofia, S.A.; Goh, W.I.; Ali, N. Innovative and sustainable green concrete—A potential review on utilization of agricultural waste. IOP Conf. Ser. Mater. Sci. Eng. 2019, 601, 012026.
- Nunes, L.J.R.; Loureiro, L.M.E.F.; Sá, L.C.R.; Silva, H.F.C. Sugarcane industry waste recovery: A case study using thermochemical conversion technologies to increase sustainability. Appl. Sci. 2020, 10, 6481.
- FAOSTAT Statistical Yearbook, World Food and Agriculture. 2021. Available online: https://www.fao.org/3/cb4477en/cb4477en.pdf (accessed on 4 July 2022).
- OECD-FAO Agricultural Outlook 2020–2029. Available online: https://www.oecd-ilibrary.org/sites/3736a600-en/index.html?itemId=/content/component/3736a600-en#section-d1e18381 (accessed on 4 July 2022).
- Faostat 2022. Sugarcane Production in 2020, Crops/Regions/World List/Production Quantity (Pick Lists). UN Food and Agriculture Organization, Corporate Statistical Database. Available online: https://www.fao.org/faostat/en/#data/QC (accessed on 4 July 2022).
- Amini, Z.; Self, R.; Strong, J.; Speight, R.; O’Hara, I.; Harrison, M.D. Valorization of sugarcane biorefinery residues using fungal biocatalysis. Biomass Convers. Biorefinery 2021, 12, 997–1011.
- Kim, M.; Day, D.F. Composition of sugar cane, energy cane, and sweet sorghum suitable for ethanol production at Louisiana sugar mills. J. Ind. Microbiol. Biotechnol. 2011, 38, 803–807.
- Boussarsar, H.; Roge, B.; Mathlouthi, M. Optimization of sugarcane bagasse conversion by hydrothermal treatment for the recovery of xylose. Bioresour. Technol. 2009, 100, 6537–6542.
- Fito, J.; Tefera, N.; Van Hulle, S.W.H. Sugarcane biorefineries wastewater: Bioremediation technologies for environmental sustainability. Chem. Biol. Technol. Agric. 2019, 6, 1–13.
- Ometto, A.R.; Hauschild, M.Z.; Roma, W.N.L. Lifecycle assessment of fuel ethanol from sugarcane in Brazil. Int J. Life Cycle Assess 2009, 14, 236–247.
- Martinelli, L.; Filoso, S.; Aranha, C.D.B.; Ferraz, S.F.B.; Andrade, T.M.B.; Ravagnani, E.D.C. Water use in sugar and ethanol industry in the State of Sao Paulo (Southeast Brazil). J. Sustain. Bioenergy Syst. 2013, 3, 135–142.
- Jugwanth, Y.; Sewsynker-Sukai, Y.; Gueguim Kana, E.B. Valorization of sugarcane bagasse for bioethanol production through simultaneous saccharification and fermentation: Optimization and kinetic studies. Fuel 2020, 262, 116552.
- Smithers, J. Review of sugarcane trash recovery systems for energy cogeneration in South Africa. Renew. Sustain. Energy Rev. 2014, 32, 915–925.
- Meghana, M.; Shastri, Y. Sustainable valorization of sugar industry waste: Status, opportunities, and challenges. Bioresour. Technol. 2020, 303, 122929.
- Pierossi, M.; Bernhardt, H.W.; Funke, T. Sugarcane leaves and tops: Their current use for energy and hurdles to be overcome, particularly in South Africa, for greater utilisation. Proc. Annu. Congr. S. Afr. Sugar Technol. Assoc. 2016, 89, 350–360.
- Balakrishnan, M.; Batra, V.S. Valorization of solid waste in sugar factories with possible applications in India: A review. J. Environ. Manag. 2011, 92, 2886–2891.
- Chandel, A.K.; da Silva, S.S.; Carvalho, W.; Singh, O.V. Sugarcane bagasse and leaves: Foreseeable biomass of biofuel and bio-products. J. Chem. Technol. Biotechnol. 2012, 87, 11–20.
- Gómez, E.O.; Souza, R.T.G.; Rocha, G.J.M.; Almeida, E.; Cortez, L.A.B. A palha de cana-de-açúcar como matéria-prima para processos de segunda geração. In Bioetanol de Cana de Açúcar; Cortez, L.A.B., Ed.; Edgard Bleucher: São Paulo, Brazil, 2010; pp. 636–659.
- Nakhla, D.A.; El Haggar, S. A proposal to environmentally balanced sugarcane industry in Egypt. Int. J. Agric. Policy Res. 2014, 2, 321–328.
- Singh, P.; Suman, A.; Tiwari, P.; Arya, N.; Gaur, A.; Shrivastava, A.K. Biological pretreatment of sugarcane trash for its conversion to fermentable sugars. World J. Microbiol. Biotechnol. 2008, 24, 667–673.
- Mahimairaja, S.; Dooraisamy, P.; Lakshmanan, A.; Rajannan, G.; Udayasoorian, C.; Natarajan, S. Composting Technology and Organic Waste Utilization in Agriculture; A.E. Publications: Coimbatore, India, 2008.
- Nunes, L.; Matias, J.; Catalão, J. A review on torrefied biomass pellets as a sustainable alternative to coal in power generation. Renew. Sustain. Energy Rev. 2014, 40, 153–160.
- Clauser, N.M.; Gutiérrez, S.; Area, M.C.; Felissia, F.E.; Vallejos, M.E. Small-sized biorefineries as strategy to add value to sugarcane bagasse. Chem. Eng. Res. Des. 2016, 107, 137–146.
- Rabelo, S.C.; Carrere, H.; Maciel Filho, R.; Costa, A.C. Production of bioethanol, methane and heat from sugarcane bagasse in a biorefinery concept. Bioresour. Technol. 2011, 102, 7887–7895.
- Costa, S.M.; Aguiar, A.; Luz, S.M.; Pessoa, A.; Costa, S.A. Sugarcane straw and its cellulosic fraction as raw materials for obtainment of textile fibers and other bioproducts. Polysaccharides 2014, 513–533.
- Monteiro, S.N.; Candido, V.S.; Braga, F.O.; Bolzan, L.T.; Weber, R.P.; Drelich, J.W. Sugarcane bagasse waste in composites for multilayered armor. Eur. Polym. J. 2016, 78, 173–185.
- Shafiq, N.; Hussein, A.A.E.; Nuruddin, M.F.; Al Mattarneh, H. Effects of sugarcane bagasse ash on the properties of concrete. Proc. Inst. Civ. Eng. 2018, 171, 123–132.
- Khoo, R.Z.; Chow, W.S.; Ismail, H. Sugarcane bagasse fiber and its cellulose nanocrystals for polymer reinforcement and heavy metal adsorbent: A review. Cellulose 2018, 25, 4303–4330.
- Sahu, O. Assessment of sugarcane industry: Suitability for production, consumption, and utilization. Ann. Agrar. Sci. 2018, 16, 389–395.
- Alves-Rezende, C.; de Lima, M.A.; Maziero, P.; Ribeiro de Azevedo, E.; Garcia, W.; Polikarpov, I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels 2011, 4, 54–72.
- Monteiro, S.N.; Lopes, F.P.D.; Barbosa, A.P.; Bevitori, A.B.; Silva, I.L.A.; Costa, L.L. Natural lignocellulosic fibers as engineering materials—An overview. Metall. Mater. Trans. A 2011, 42, 2963–2974.
- Bhat, S.A.; Singh, J.; Vig, A.P. Management of sugar industrial wastes through vermitechnology. Int. Lett. Nat. Sci. 2016, 55, 35–43.
- Sarker, T.C.; Azam, S.M.G.G.; Bonanomi, G. Recent advances in sugarcane industry solid by-products valorization. Waste Biomass Valorization 2017, 8, 241–266.
- Munagala, M.; Shastri, Y.; Nalawade, K.; Konde, K.; Patil, S. Life cycle and economic assessment of sugarcane bagasse valorization to lactic acid. Waste Manag. 2021, 126, 52–64.
- Martinez-Hernandez, E.; Amezcua-Allieri, M.A.; Sadhukhan, J.; Aburto, J. Sugarcane bagasse valorization strategies for bioethanol and energy production. In Sugarcane; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018; Chapter 4.
- Chandel, A.K.; Antunes, F.A.; Terán-Hilares, R.; Cota, J.; Ellilä, S.; Silveira, M.H.; dos Santos, J.C.; da Silva, S.S. Bioconversion of hemicellulose into ethanol and value-added products: Commercialization, trends, and future opportunities. In Advances in Sugarcane Biorefinery. Technologies, Commercialization, Policy Issues and Paradigm Shift for Bioethanol and By-Products; Elsevier B.V.: Amsterdam, The Netherlands, 2018; pp. 97–134.
- Cardona, C.A.; Quintero, J.A.; Paz, I.C. Production of bioethanol from sugarcane bagasse: Status and perspectives. Bioresour. Technol. 2010, 101, 4754–4766.
- Contreras-Lisperguer, R.; Batuecas, E.; Mayo, C.; Díaz, R.; Pérez, F.J.; Springer, C. Sustainability assessment of electricity cogeneration from sugarcane bagasse in Jamaica. J. Clean. Prod. 2018, 200, 390–401.
- Iryani, D.; Hirajima, T.; Kumagai, S.; Nonaka, M.; Sasaki, K. Overview of Indonesian sugarcane industry and utilization of its solid waste. In Proceedings of the Annual Fall Meeting of the Mining and Materials Processing Institute of Japan (MMIJ), Akita, Japan, 14–16 October 2012.
- Hofsetz, K.; Silva, M.A. Brazilian sugarcane bagasse: Energy and non-energy consumption. Biomass Bioenergy 2012, 46, 564–573.
- Arif, E.; Clark, M.W.; Lake, N. Sugar cane bagasse ash from a high efficiency co-generation boiler: Applications in cement and mortar production. Constr. Build. Mater. 2016, 128, 287–297.
- Alves, M.; Ponce, G.H.; Silva, M.A.; Ensinas, A.V. Surplus electricity production in sugarcane mills using residual bagasse and straw as fuel. Energy 2015, 91, 751–757.
- Carriel Schmitt, C.; Moreira, R.; Cruz Neves, R.; Richter, D.; Funke, A.; Raffelt, K.; Grunwaldt, J.D.; Dahmen, N. From agriculture residue to upgraded product: The thermochemical conversion of sugarcane bagasse for fuel and chemical products. Fuel Process. Technol. 2020, 197, 106199.
- Quereshi, S.; Naiya, T.K.; Mandal, A.; Dutta, S. Residual sugarcane bagasse conversion in India: Current status, technologies, and policies. In Biomass Conversion and Biorefinery; Springer Professional: Hong Kong, China, 2020.
- Turdera, M.V. Energy balance, forecasting of bioelectricity generation and greenhouse gas emission balance in the ethanol production at sugarcane mills in the state of Mato Grosso do Sul. Renew. Sustain. Energy Rev. 2013, 19, 582–588.
- Ravindranath, N.H.; Balachandra, P.; Dasappa, S.; Usha, R.K. Bioenergy technologies for carbon abatement. Biomass Bioenergy 2006, 30, 826–837.
- Texteira, S.R.; Pena, A.F.V.; Miguel, A.G. Briquetting of charcoal from sugarcane bagasse fly ash (SCBFA) as an alternative fuel. Waste Manag. 2010, 30, 804–807.
- Sales, A.; Lima, S.A. Use of Brazilian sugarcane bagasse ash in concrete as sand replacement. Waste Manag. 2010, 30, 1114–1122.
- Iyer, P.V.R.; Rao, T.R.; Grover, P.D. Biomass Thermo-Chemical Characterization, 3rd ed.; Indian Institute of Technology: New Delhi, India, 2002.
- Xu, Q.; Ji, T.; Gao, S.-J.; Yang, Z.; Wu, N. Characteristics and applications of sugar cane bagasse ash waste in cementitious materials. Materials 2018, 12, 39.
- Onchieku, J.; Chikamai, B.; Rao, M. Optimum parameters for the formulation of charcoal briquettes using bagasse and clay as binder. Eur. J. Sustain. Dev. 2012, 1, 477.
- Setter, C.; Sanchez Costa, K.L.; Pires de Oliveira, T.J.; Farinassi Mendes, R. The effects of kraft lignin on the physicomechanical quality of briquettes produced with sugarcane bagasse and on the characteristics of the bio-oil obtained via slow pyrolysis. Fuel Process. Technol. 2020, 210, 106561.
- Kazmi, S.M.S.; Abbas, S.; Saleem, M.A.; Munir, M.J.; Khitab, A. Manufacturing of sustainable clay bricks: Utilization of waste sugarcane bagasse and rice husk ashes. Constr. Build. Mater. 2016, 120, 29–41.
- Abdulkadir, T.; Oyejobi, D.; Lawal, A. Evaluation of sugarcane bagasse ash as a replacement for cement in concrete works. Acta Tech. Corviniensis-Bull. Eng. 2014, 3, 71–76.
- de Siqueira, A.A.; Cordeiro, G.C. Sustainable cements containing sugarcane bagasse ash and limestone: Effects on compressive strength and acid attack of mortar. Sustainability 2022, 14, 5683.
- Faria, K.C.P.; Gurgel, R.F.; Holanda, J.N.F. Recycling of sugarcane bagasse ash waste in the production of clay bricks. J. Environ. Manag. 2012, 101, 7–12.
- Anjos, M.A.S.; Araújo, T.R.; Ferreira, R.L.S.; Farias, E.C.; Martinelli, A.E. Properties of self-leveling mortars incorporating a high-volume of sugar cane bagasse ash as partial Portland cement replacement. J. Build. Eng. 2020, 32, 101694.
- Kulkarni, P.; Ravekar, V.; Rao, P.R.; Waigokar, S.; Hingankar, S. Recycling of waste HDPE and PP plastic in preparation of plastic brick and its mechanical properties. Clean Mat. 2022, 4, 100113.
- Tripathy, A.; Acharya, P.K. Characterization of bagasse ash and its sustainable use in concrete as a supplementary binder—A review. Constr. Build. Mater. 2022, 322, 126391.
- Andrew, R.M. Global CO2 emissions from cement production. Earth Syst. Sci. Data 2018, 10, 195–217.
- Kusumaningtyas, R.D.; Hartanto, D.; Rohman, H.A.; Mitamaytawati; Qudus, N.; Daniyanto. Valorization of sugarcane-based bioethanol industry waste (vinasse) to organic fertilizer. In Valorisation of Agro-industrial Residues—Volume II: Non-Biological Approaches; Zakaria, Z., Aguilar, C., Kusumaningtyas, R., Binod, P., Eds.; Applied Environmental Science and Engineering for a Sustainable Future; Springer: Cham, Switzerland, 2020.
- Hoarau, J.; Caro, Y.; Grondin, I.; Petit, T. Sugarcane vinasse processing: Toward a status shift from waste to valuable resources: A review. J. Water Process. Eng. 2018, 24, 11–25.
- Reis, C.E.R.; Bento, H.B.S.; Alves, T.M.; Carvalho, A.K.F.; De Castro, H.F. Vinasse treatment within the sugarcane-ethanol industry using ozone combined with anaerobic and aerobic microbial processes. Environments 2019, 6, 5.
- Moraes, B.S.; Zaiat, M.; Bonomi, A. Anaerobic digestion of vinasse from sugarcane ethanol production in Brazil: Challenges and perspectives. Renew. Sustain. Energy Rev. 2015, 44, 888–903.
- Carpanez, T.G.; Moreira, V.R.; Assis, I.R.; Amaral, M.C.S. Sugarcane vinasse as organo-mineral fertilizers feedstock: Opportunities and environmental risks. Sci. Total Environ. 2022, 832, 154998.
- Christofoletti, C.A.; Escher, J.P.; Correia, J.E.; Marinho, J.F.U.; Fontanetti, C.S. Sugarcane vinasse: Environmental implications of its use. Waste Manag. 2013, 33, 2752–2761.
- Moreira, L.C.; Borges, P.O.; Cavalcante, R.M.; Young, A.F. Simulation and economic evaluation of process alternatives for biogas production and purification from sugarcane vinasse. Renew. Sustain. Energy Rev. 2022, 163, 112532.
- Kiani, M.K.D.; Parsaee, M.; Ardebili, S.M.S.; Pereda Reyes, I.; Fuess, L.T.; Karimi, K. Different bioreactor configurations for biogas production from sugarcane vinasse: A comprehensive review. Biomass Biorenergy 2022, 161, 106446.
- Ramos, L.R.; Lovato, G.; Domingues Rodrigues, J.A.; Silva, E.L. Scale-up and energy estimations of single- and two-stage vinasse anaerobic digestion systems for hydrogen and methane production. J. Clean. Prod. 2022, 349, 131459.
- Tano, F.; Valenti, L.; Failla, O.; Beltrame, E. Effects of distillery vinasses on vineyard yield and quliaty in the D.O.C. “Oltrepo Pavese Pinot Nero”—Lombardy, Italy. Water Sci. Technol. 2005, 151, 199–204.
- Santos, F.; Eichler, P.; Machado, G.; De Mattia, J.; De Souza, G. By-products of the sugarcane industry. In Sugarcane Biorefinery, Technology and Perspectives; Santos, F., Rabelo, S., De Matos, M., Eichler, P., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 21–48.
- Hashemi, S.S.; Karimi, K.; Taherzadeh, M.J. Valorization of vinasse and whey to protein and biogas through an environmental fungi-based biorefinery. J. Environ. Manag. 2022, 303, 114138.
- Yadav, R.L.; Solomon, S. Potential of developing sugarcane by-product based industries in India. Sugar Tech. 2006, 8, 104–111.
- Partha, N.; Sivasubramanian, V. Recovery of chemicals from press mud—A sugar industry waste. Indian Chem. Eng. 2006, 48, 160–163.
- Ochoa-George, P.A.; Eras, J.J.; Gutierrez, A.S.; Hens, L.; Vandecasteele, C. Residue from sugarcane juice filtration (filter cake): Energy use at the sugar factory. Waste Biomass Valor. 2010, 1, 407–413.
- Sen, B.; Chandra, T.S. Chemolytic and solid-state spectroscopic evaluation of organic matter transformation during vermicomposting of sugar industry wastes. Bioresour. Technol. 2007, 98, 1680–1683.
- Casas, L.; Hernández, Y.; Mantell, C.; Casdelo, N.; Ossa, E.M. Filter cake oil-wax as raw material for the production of biodiesel: Analysis of the extraction process and the transesterification reaction. J. Chem. 2015, 2015, 946462.
- Jamil, M.; Qasim, M.; Zia, M.S. Utilization of press mud as organic amendment to improve physico-chemical characteristics of calcareous soil under two legume crops. J. Chem. Soc. Pak. 2008, 30, 577–583.
- Fantaye, A.; Fanta, A.; Melesse, A.M. Effect of filter press mud application on nutrient availability in aquert and fluvent soils of Wonji/Shoa sugarcane plantation of Ethiopia. In Landscape Dynamics, Soils and Hydrological Processes in Varied Climates; Melesse, A., Abtew, W., Eds.; Springer Geography: Berlin/Heidelberg, Germany, 2016; pp. 549–563.
- Rakkiyappan, P.; Thangavelu, S.; Malathi, R.; Radhamani, R. Effect of biocompost and enriched pressmud on sugarcane yield and quality. Sugar Tech. 2001, 3, 92–96.
- Sarangi, B.K.; Mudliar, S.N.; Bhatt, P.; Kalve, S.; Chakrabarti, T.; Pandey, R.A. Compost from sugar mill press mud and distillery spent wash for sustainable agriculture. Dyn. Soil Dyn. Plant 2009, 2, 35–49.
- Nenciu, F.; Stanciulescu, I.; Vlad, H.; Gabur, A.; Turcu, O.L.; Apostol, T.; Vlădut, V.N.; Cocârță, D.; Stan, C. Decentralized processing performance of fruit and vegetable waste discarded from retail, using an automated thermophilic composting technology. Sustainability 2022, 14, 2835.
- Kumar, V.; Chopra, A.K. Fertigation with agro-residue based paper mill effluent on a high yield spinach variety. Int. J. Veg. Sci. 2015, 21, 69–97.
- Sarwar, G.; Schmeisky, H.; Hussain, N.; Muhammad, S.; Ibrahim, M.; Safdar, E. Improvement of soil physical and chemical properties with compost application in rice-wheat cropping system. Pak. J. Bot. 2008, 40, 275–282.
- Keshavanath, P.; Shivanna Gangadhara, B. Evaluation of sugarcane by-product press mud as a manure in carp culture. Bioresour. Technol. 2006, 97, 628–634.
- Gupta, N.; Tripathi, S.; Balomajumder, C. Characterization of press mud: A sugar industry waste. Fuel 2011, 90, 389–394.
- Ansari, K.B.; Gaikar, V.G. Pressmud as an alternate resource for hydrocarbons of green engineering. Environ. Sci. Technol. 2014, 37, 94–101.
- El Haggar, S.; El Gowini, M.M.; Nemerow, N.L.; Veziroglu, T.N. Environmentally balanced industrial complex for the cane sugar industry in Egypt. In Proceedings of the International Hydrogen Energy Congress and Exhibition IHEC, Istanbul, Turkey, 13–15 July 2005.
- Asaithambi, P.; Matheswaran, M. Electrochemical treatment of simulated sugar industrial effluent: Optimization and modeling using a response surface methodology. Arab. J. Chem. 2016, 9, S981–S987.
- Zver, L.Ž.; Glavič, P. Water minimization in process industries: Case study in beet sugar plant. Resour Conserv Recycl. 2005, 43, 133–145.
- Prakash, S. Sugar mill effluent induced histological changes in intestine of Channa punctatus. Ind. J. Biol. Stud. Res. 2011, 1, 32–35.
- Doble, M.; Kruthiventi, A.K. Industrial examples. In Green Chemistry and Engineering; Academic Press: Cambridge, MA, USA, 2007; Chapter 9; pp. 245–296.
- Guven, G.; Perendeci, A.; Tanylac, A. Electrochemical treatment of simulated beet sugar factory wastewater. Chem. Eng. J. 2009, 151, 149–159.
- Baraniya, C.; Jodhi, C. Performance evaluation of effluent treatment plant for sugar mill effluent. Int. J. Creat. Res. Thoughts IJCRT 2018, 6, 1492–1499.
- Memon, A.R.; Soomro, S.A.; Ansari, A.K. Sugar industry effluent—Characteristics and chemical analysis. J. App. Em. Sc. 2006, 1, 152–157.
- Sahu, O.P.; Chaudhari, P.K. Electrochemical treatment of sugar industry wastewater: COD and color removal. J. Electroanal. Chem. 2015, 739, 122–129.
- Lappa, K.; Kandylis, P.; Bekatorou, A.; Bastas, N.; Klaoudatos, S.; Athanasopoulos, N.; Kanellaki, M.; Koutinas, A.A. Continuous acidogenesis of sucrose, raffinose and vinasse using mineral kissiris as promoter. Bioresour. Technol. 2015, 188, 43–48.
- Teclu, D.; Tivchev, G.; Laing, M.; Wallis, M. Determination of the elemental composition of molasses and its suitability as carbon source for growth of sulphate-reducing bacteria. J. Hazard. Mater. 2009, 161, 1157–1165.
- Amorim, H.V.; Baso, L.C.; Lopes, M.L. Sugar cane juice and molasses, beet molasses and sweet sorghum: Composition and usage. In Alcohol Textbook; Nothingham University Press: Trumpton Nottingham, UK, 2003; pp. 39–46.
- Clarke, M.A. Syrups. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Luiz, T., Paul, F., Eds.; Elsevier Science: Amsterdam, The Netherlands, 2003.
- Khan, S.; Lu, F.; Kashif, M.; Shen, P. Multiple effects of different nickel concentrations on the stability of anaerobic digestion of molasses. Sustainability 2021, 13, 4971.
- Raharja, R.; Murdiyatmo, U.; Sutrisno, A.; Wardani, A.K. Bioethanol production from sugarcane molasses by instant dry yeast. IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012076.
- Basso, L.C.; Basso, T.O.; Rocha, S.N. Ethanol production in Brazil: The industrial process and its impact on yeast fermentation. Biofuel Prod. Recent Dev. Prospect. 2011, 1530, 85–100.
- Kartini, A.M.; Dhokhikah, Y. Bioethanol production from sugarcane molasses with simultaneous saccharification and fermentation (SSF) Method using Saccaromyces cerevisiae-Pichia stipitis consortium. IOP Conf. Ser. Earth Environ. Sci. 2018, 207, 012061.
- Jayanti, A.N.; Sutrisno, A.; Wardani, A.K.; Murdiyatmo, U. Bioethanol production from sugarcane molasses by instant dry yeast (effect of pretreatment and fermentation temperature). IOP Conf. Ser. Earth Environ. Sci. 2019, 230, 012102.
- Parascanu, M.M.; Sanchez, N.; Sandoval-Salas, F.; Mendez Carreto, C.; Soreanu, G.; Sanchez-Silva, L. Environmental and economic analysis of bioethanol production from sugarcane molasses and agave juice. Environ. Sci Pollut. Res. 2021, 28, 64374–64393.
- Afschar, A.S.; Vaz Rossell, C.E.; Schaller, K. Bacterial conversion of molasses to acetone and butanol. Appl. Microbiol. Biotechnol. 1990, 34, 168–171.
- Nikodinovic-Runic, J.; Guzik, M.; Kenny, S.T.; Babu, R.; Werker, A.; O’Connor, K.E. Carbon-rich wastes as feedstocks for biodegradable polymer (polyhydroxyalkanoate) production using bacteria. Adv. Appl. Microbiol. 2013, 84, 139–200.
- Nicol, D.A. Rum. In Fermented Beverage Production; Lea, A.G.H., Piggott, J.R., Eds.; Springer: Boston, MA, USA, 2003; pp. 263–287.
- Gandiglio, M.; Lanzini, A. Biogas resource potential and technical exploitation. In Comprehensive Renewable Energy, 2nd ed.; Sayigh, A., Ed.; Elsevier B.V.: Amsterdam, The Netherlands, 2022.
- Portilla, O.M.; Espinosa, V.; Jarquin, L.; Salinas, A.; Velazquez, G.; Vazquez, M. Sugar cane molasses as culture media component for microbial transglutaminase production. Indian J. Biotechnol. 2017, 16, 419–425.
- Oliveira Lino, F.S.; Basso, T.O.; Sommer, M.O.A. A synthetic medium to simulate sugarcane molasses. Biotechnol. Biofuels 2018, 11, 221.
- Vignesh Kumar, B.; Muthumari, B.; Kavitha, M.; John Praveen Kumar, J.K.; Thavamurugan, S.; Arun, A.; Jothi Basu, M. Studies on optimization of sustainable lactic acid production by Bacillus amyloliquefaciens from sugarcane molasses through microbial fermentation. Sustainability 2022, 14, 7400.
