Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The synthesis of polyhydroxyalkanoates (PHAs), a bioplastic that can be used to replace traditional (petrol-based) plastics, is an important focus in today’s politically and environmentally conscious society.
- polyethylene (PE)
- polypropylene (PP)
- polyhydroxyalkanoate (PHA)
- PHAS
- bioplastic
- biotechnology
1. Introduction
The synthesis of polyhydroxyalkanoates (PHAs), a bioplastic that can be used to replace traditional (petrol-based) plastics, is an important focus in today’s politically and environmentally conscious society. PHAs are part of a group of organic polymers containing 3-, 4-, 5-, and 6-hydroxyalkanoic acids that are biocompatible, 100% biodegradable, and nontoxic to the environment [1,2]. PHAs can be considered a greener alternative to synthetic plastic compounds, and they can be produced by plants and various strains of bacteria (as documented later). In addition, these bioplastics can be generated through microbial fermentation using waste materials which could offer more sustainability in a closed-cycle system of carbon materials [2]. Some of the waste materials that have been used to make PHAs have included used synthetic plastics, such as PE, PS, and PP [2,3,4]. From 2022, by changing the co-monomer type and distribution in PHAs, the properties can be considered to be comparable with seven of the most profitable crude-oil-based plastics, which is estimated to be 230 million tons of plastic per year [5]. There have also been global policies put in place and capacity expansions for the next 5 years for over 1.4 million tons, so there is a lot of encouragement for the industry to adopt biomaterials [5,6]. It is also predicted that 12,000 million tons of plastic waste will be added to landfills or the natural environment by 2050 [7]. Figure 1 shows a timeline of milestones related to bioplastic development over the last hundred years.
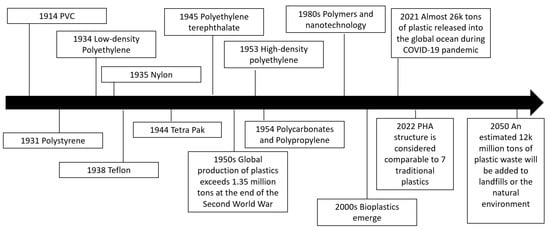
Figure 1. Milestones over the last century summarizing how bioplastics fit into the plastics industry.
Research projects around the world have focused on using microbes to break down some the most persistent types of plastics found in the environment. Currently, the most common way plastics are disposed of is by incineration, mechanical and chemical recycling, and the relatively cheap method of landfill sites [8]. However, all of these methods have their disadvantages; landfill occupies too much land space, and incineration creates secondary pollutants, such as dioxins and carbon monoxide. Even though mechanical recycling has become the main technique for refuge plastic, the chemical properties are usually compromised via processing, which results in reduced commercial value [9].
Chemical recycling is known to be able to recover monomers from plastic waste, but its success depends heavily on the efficiency of catalysts [9]. With up to 79% of waste plastic being discarded into landfills (or the surrounding environment), there is a huge requirement for novel recycling methodologies, and bioconversion is one possible answer [4,10,11]. The transfer of current feedstocks could be smoother if the true economical value (including the carbon footprint of products and practical benefits) were considered in detail. The efficiency of biotechnological methods can also be further improved using metabolic engineering, which could help achieve the aims of the internationally agreed Paris Agreement, a treaty on climate change [10]. Moreover, the recently estimated impact of the COVID-19 pandemic on plastic discharge indicates that around 8.4 million tons of pandemic-associated plastic waste was generated from 193 countries, as of 23 August 2021, and over 25.9 thousand tons were released into the global ocean [12]. With these issues in mind, the diagram in Figure 2 displays a possible system for generating biomaterials, such as PHAs from waste plastics via fermentation.
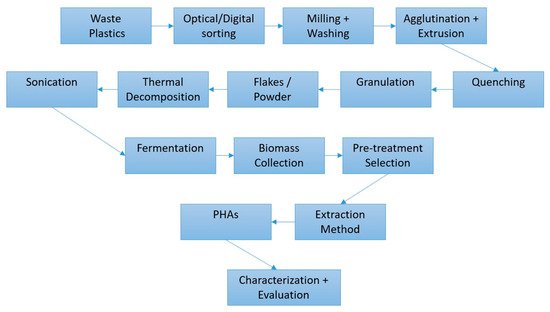
Figure 2. Potential operation stages for waste plastic material for the bio-generation of PHA bioplastics, adapted from [3]. The ‘Pre-treatment Selection’ of ‘Biomass’ process could include methods such as ionic liquid soaking, sonication, glass sphere mixing, and blade/pressure homogenization. Every procedure such as this in today’s economy should end with an evaluation step to ascertain any shortcomings and potential investigation avenues.
Elements of this kind of system exist in parts, such as the optical scanning and separation and sorting of plastics; the innovation would be in having all of these sections interlinked. In some cases, processes such as milling, agglutination, or sonication would have to be selected on the basis of the target material’s properties. After thermal pre-treatments of PS or PE, where oxygenated groups were incorporated into the unsaturated carbon backbone, sonication was found to greatly increase the mixing of plastics with the growth media for fermentation [2,3,4]. Due to the nature of microbial cultivation, requiring time (normally 48hrs or more) and optimal growth conditions (usually ranging from 30 to 37 °C at 50 to 150 rpm), a lot of energy is needed [4,10]. This is perhaps the major bottleneck for such a system, which is why the choice of micro-organisms used is so important. Additionally, any biological PHA generation has the often-unreported issue of difficulty in controlling the precise purity and biopolymer composition. Moreover, there is evidence showing that the extraction processes can alter the PHA-polymer structure when conventional chemicals (such as chloroform) and Soxhlet extraction are used [13]. The extraction yields and the PHA properties can also be affected. Data revealed the two different extraction methods alter the crystallization degree and the chemical composition. When pure bioplastic is required, pre-treatments such as homogenization provided a 15% more extractive yield than the others, especially at high pressures, which also improved the visual appearance (transparency and clearness), thermal stability, and mechanical performances, which is ideal for medical grade PHAs [13]. For packaging (the major application of PHAs), these polymers have already been proposed to effectuate a significant shift in the industry, which currently utilizes almost 40% of plastics created [14].
2. Value-Added Bioplastic Synthesis
The issues of many of the current ways of managing waste plastics (burning and landfill) can often lead to the creation of secondary pollution events. The techniques featured in Figure 2 employ large amounts of energy, which are generally not environmentally friendly or financially viable. Due to poor recycling strategies globally and the durable properties of plastic materials, serious environmental issues, such as oceanic and soil pollution are happening [20]. For these reasons certain plastics have gained attention as carbon sources, especially those that need milder temperatures and less energy consumption for their pre-treatments.
PHAs have attracted a lot of attention mainly due to their similarities to petrochemical polymers, such as those mentioned previously, which makes them a sustainable alternative for a wide range of uses. They can be dissolved in chlorinated solvents and PHAs show a range of properties, from brittle thermoplastics to gummy elastomers, depending on the nature of the fermentation conditions and the carbon-source metabolized by the PHA producer organism [58,59,60]. The structure and the composition of the biopolymers dictate the degradation rate in the environment, and the microbes that generate PHAs cover a broad range, including both Gram positive and Gram negative bacterial strains, as shown in Table 1.
Table 1. Notable research of bioplastic production from the last 15 years on Gram-positive (+) and Gram-negative (−) bacterial strains, with their respective carbon sources and biopolymers produced. * Bio-PU a novel bio-based poly (amide urethane).
| Strain | Carbon Source | Polymer Synthesised | References |
|---|---|---|---|
| Bacillus megaterium (+) | Glucose salt medium | PHB | [61] |
| Bacillus spp. (+) | Soy molasses, nutrient broth, glucose, butyrate, valerate, hexanoate, octanoate, decanoate, 4-hydroxybutanoate, e-caprolactone | PHB, PHBV, copolymers | [61] |
| Burkholderia cepacia (−) | Palm olein, palm stearin, crude palm oil, palm kernel oil, oleic acid, xylose, levulinic acid, sugar beet molasses, sugar maple hemicellulosic hydrolysate | PHB, PHBV | [62] |
| Caryophanon latum (+) | Nutrient broth | PHA | [63] |
| Cupriavidus necator (−) | Glucose, soybean oil, waste PE, PP, PS, plastics, biodiesel by-product substrates | PHB, PHBV, PHBH, PHBHx, copolymers | [2,3,4,21,54,56,64,65] |
| Caldimonas taiwanensis (−) | Potatoe and wheat starch | PHBV | [66] |
| Bacillus odysseyi SUK3 (+) | PS plastic | PHB | [67] |
| Haloferax mediterranei (−) | Molasses and wastewater | PHBV | [68] |
| Pseudomonas umsongensis GO16 (−) | Ethylene glycol | PHA, * Bio-PU | [69] |
| Zoogloea spp. (−) | Nutrient broth (activated sludge/wastewater) | PHA | [70] |
Gram-negative Cupriavidus necator has been shown to have an accumulation yield of up to 90% cell dry weight, and, for this reason, it is the most studied microbe for PHA production [71]. The metabolic pathways used by C. necator are well-documented, both for aerobic and anaerobic conditions. In the cases of alternative carbon substrates (such as pretreated plastics), biochemical pathways I, II, and III can be selected by the organism. Figure 3 displays these pathways and how they intersect. These pathways are similar to those utilized by Bacillus megaterium, which can accumulate up to 62% cell dry weight, a different archaea species from the family Halobacteriaceae that has also been found to produce PHA biopolymers [72,73,74,75,76].
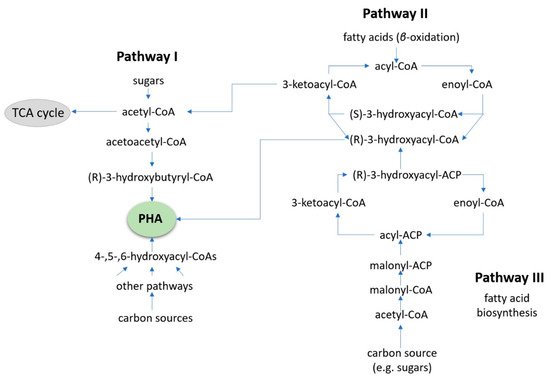
Figure 3. The combined pathways I, II, and III used by C. necator for PHA bioconversion. Similar biochemical pathways would be expected for other bacterial species capable of PHA synthesis where oxidized PS, PE, or PP waste plastic is treated as a fatty acid.
Glucose and fructose are normally processed in pathway I, generating PHB homopolymers. Fatty acids or sugars are metabolized via pathways II, III, or potentially other routes where copolymers can be produced [77,78,79]. Oxidized PE, PS, and PP particles are thought to enter the β-oxidation pathway forming acetyl-CoA that is then metabolized along pathway I, creating PHA polymers [75,76]. Both pure and mixed cultures (such as a cascade set-up with biosurfactant or hydrolase synthesizers) have the ability to make use of waste materials as feedstock to produce value-added PHAs. This approach, combined with the use of and using locally sourced refuse, could contribute to vastly reducing bioplastic expenses. PHAs are a good alternative to traditional plastics, but they have a long way to go before they can surpass them, due to their high production costs, lack of specific policies, and the downstream processing [76,79]. With that said, the next evolution in bioconversion to produce PHAs is likely to be focus towards extremophile strains. The reason for this is that they combine pure culture advantages (easier optimization of conditions), plus time can be saved by working in non-sterile conditions, simplifying the extraction and reducing the running costs.
This entry is adapted from the peer-reviewed paper 10.3390/bioengineering9090432
This entry is offline, you can click here to edit this entry!
