Highly active anti-retroviral therapy (HAART) is prescribed for HIV infection and, to a certain extent, limits the infection’s spread. Nanopharmaceuticals offer excellent treatment options for HIV infections by improving the drug potency/efficacy, lowering the dose-related toxicities, and providing active targeting options to the remote HIV reservoirs, leading to the near-total eradication of the virus. Nanopharmaceuticals offer advantages over conventional drug delivery, such as an encapsulation of the drug in nanocarriers, despite its physiochemical properties, providing long-acting treatment options and reducing the dosing because of selective targeting and improvements to the bioavailability of the hydrophobic drugs and adherence of the patients.
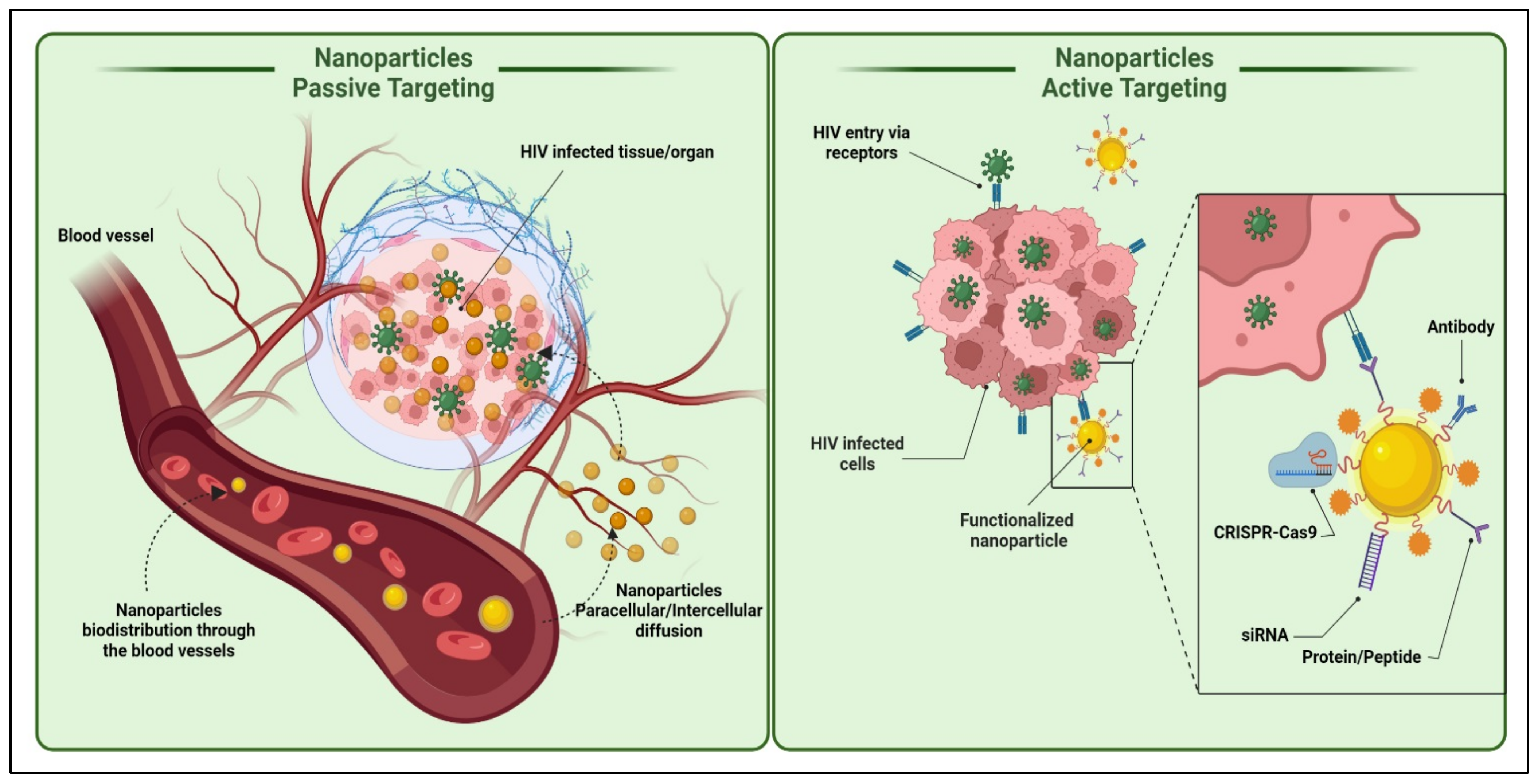
- active targeting
- HIV reservoirs
- nanomedicine
- passive targeting
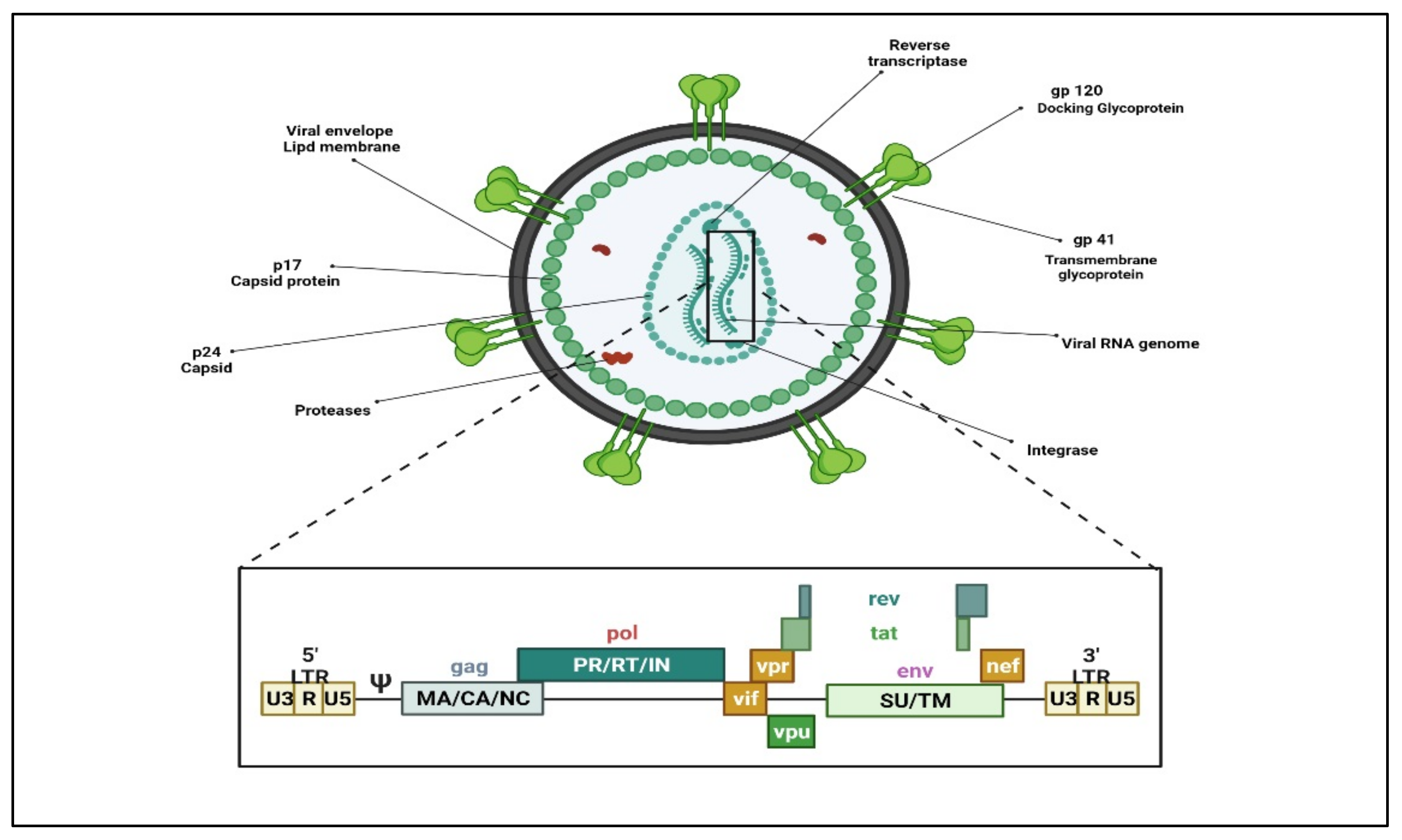
1. Human Immunodeficiency Virus (HIV)
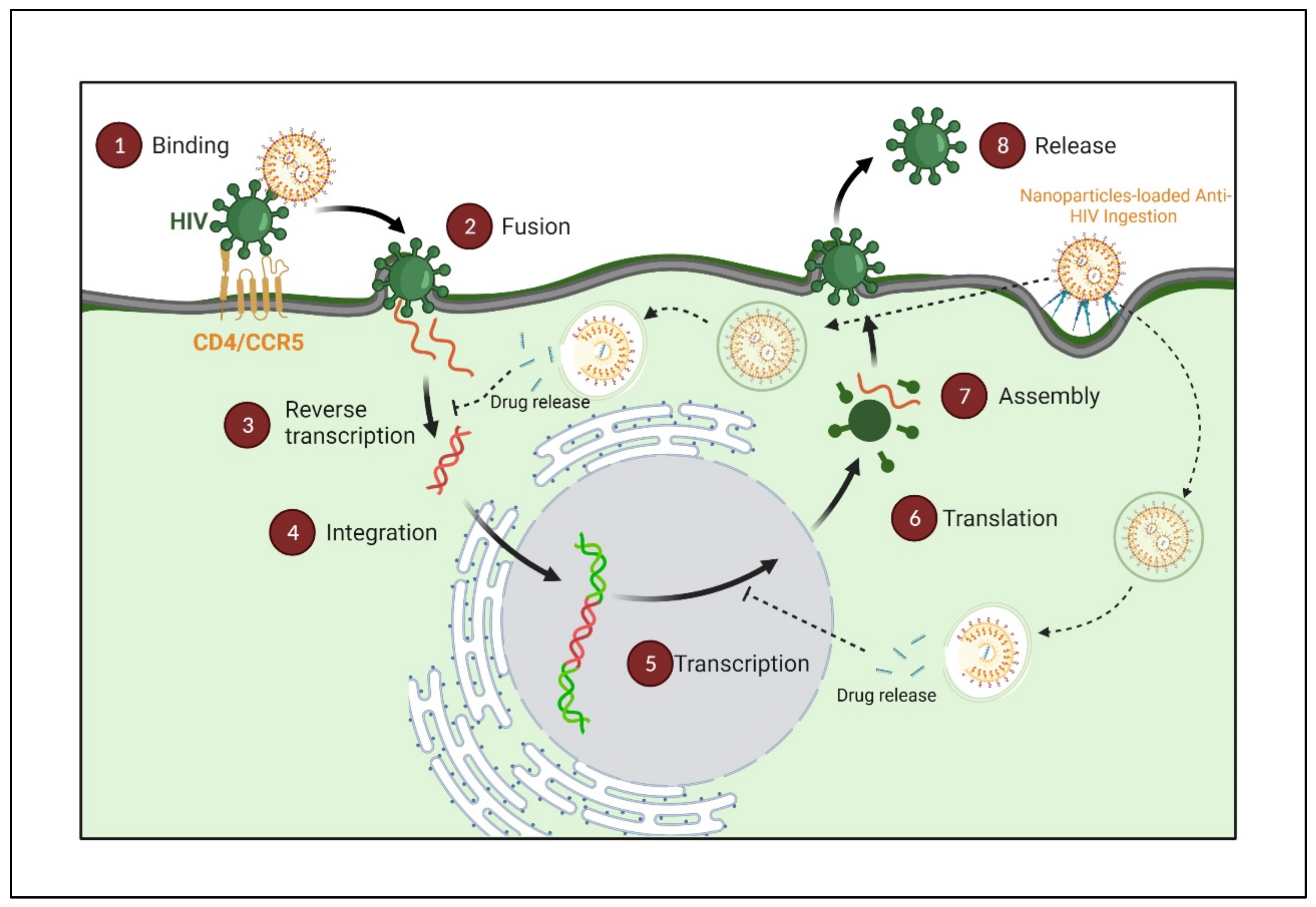
2. Nanopharmaceuticals; Novel Directions on HIV/AIDS Treatment Approaches
3. Nanoparticles Transport Approaches
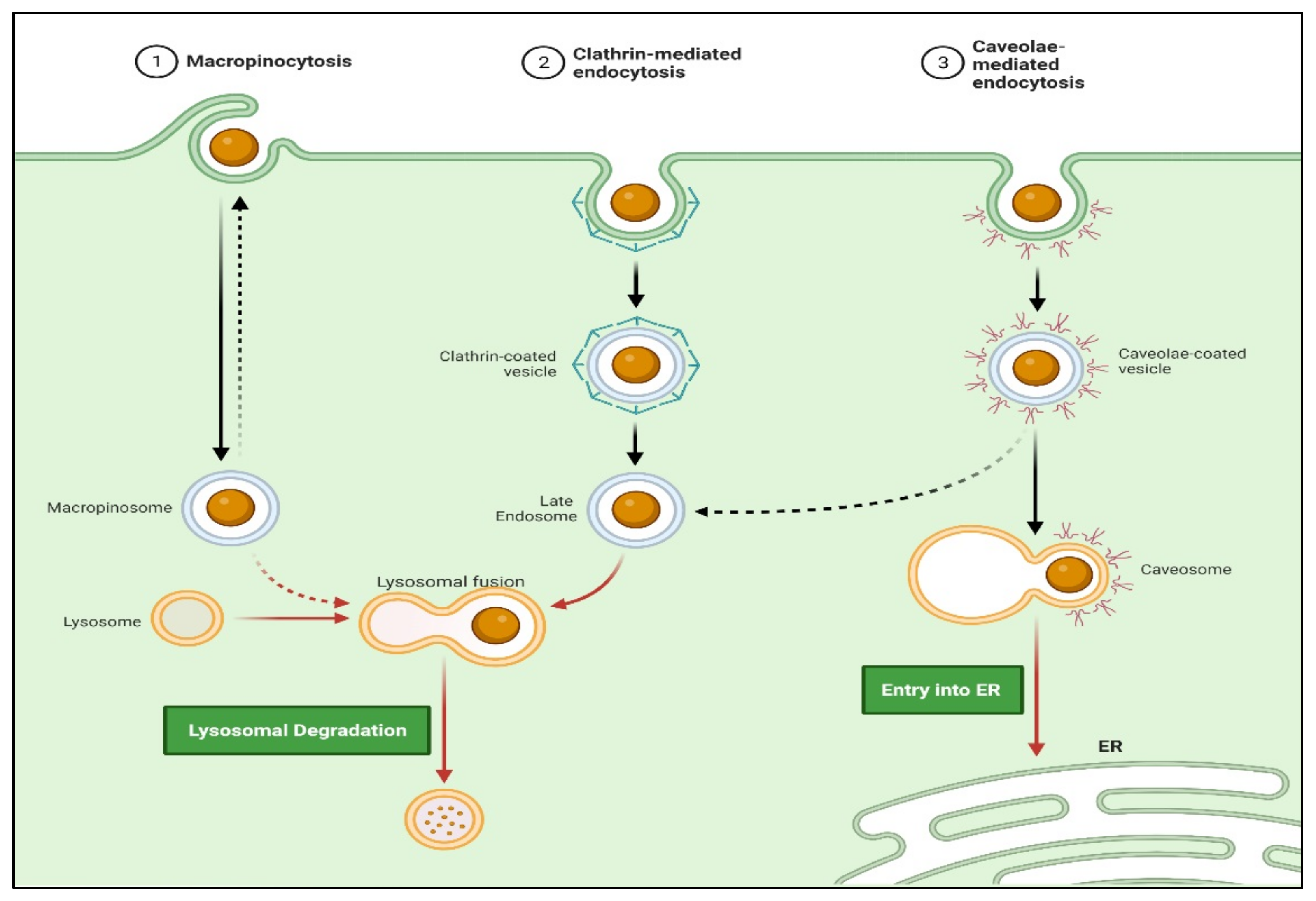
3.1. Active Transport
Stimuli-Responsive Nanocarriers
Antibody Targeted Nanocarriers
Receptor-Mediated Endocytosis (RME)
The d-Mannose Receptor Targeting
3.2. Passive Targeting
Endocytosis
Phagocytosis
4. Factors Impacting the Functionalities of Nanocarrier Targeted Delivery
4.1. Particle Size
4.2. Particle Shape
4.3. Surface Charge
4.4. Surface Hydrophobicity
5. Liposomes-Based Delivery Systems for Anti-HIV Therapeutics
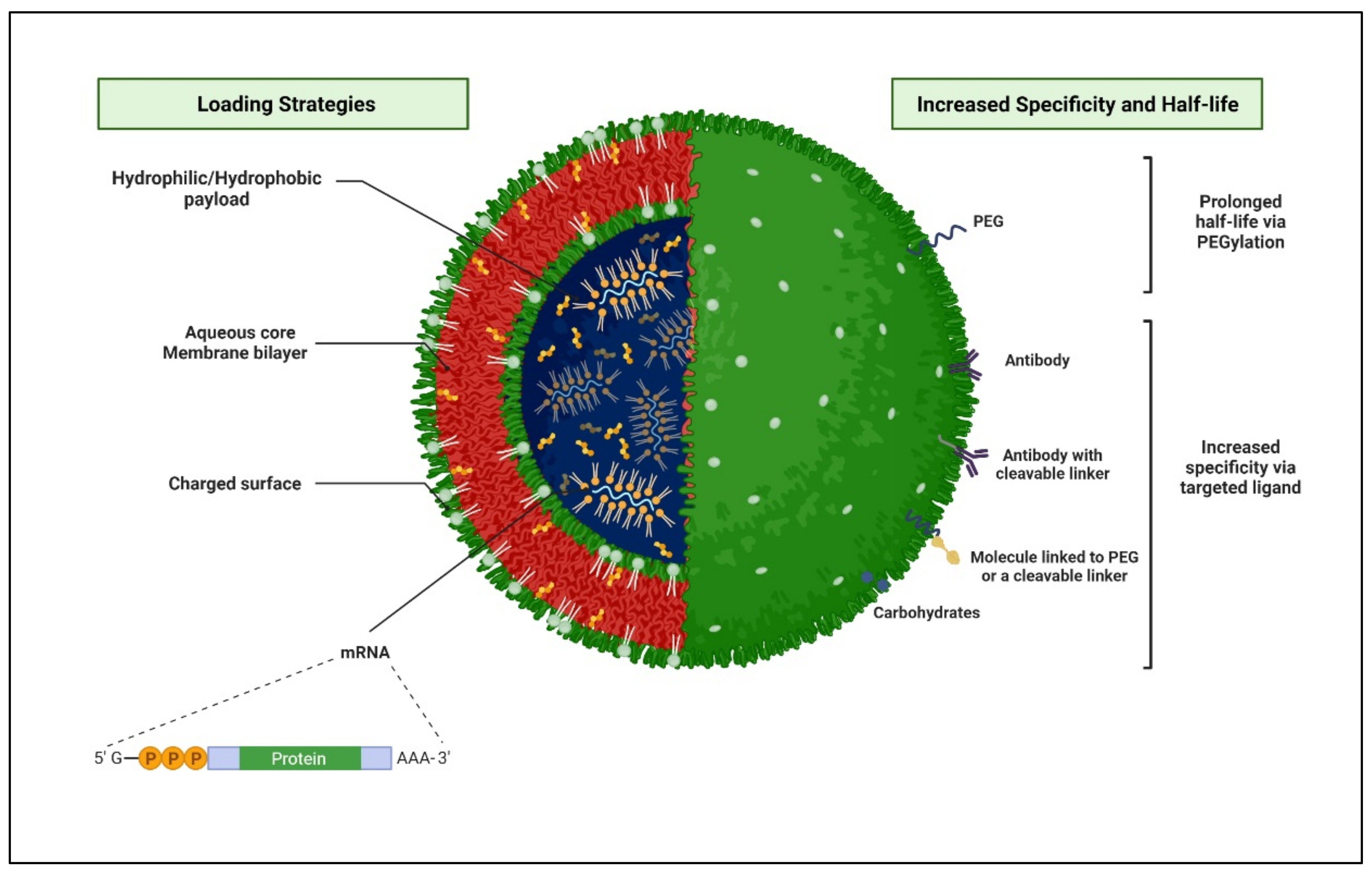
Liposomes-Based Delivery Systems of Ascorbic Acid to Increase the Bioavailability of ARTs
6. Nanotechnological Advantages for Effective Anti-HIV Therapy
This entry is adapted from the peer-reviewed paper 10.3390/polym14153090
References
- Dalvi, B.R.; Siddiqui, E.A.; Syed, A.S.; Velhal, S.M.; Ahmad, A.; Bandivdekar, A.B.; Devarajan, P.V. Devarajan, P. Nevirapine Loaded Core Shell Gold Nanoparticles by Double Emulsion Solvent Evaporation: In Vitro and In Vivo Evaluation. Curr. Drug Deliv. 2016, 13, 1071–1083.
- Unaids.Org Global HIV & AIDS Statistics—2018 Fact Sheet|UNAIDS. Available online: http://www.unaids.org/en/resources/fact-sheet (accessed on 1 June 2022).
- Mahajan, K.; Rojekar, S.; Desai, D.; Kulkarni, S.; Vavia, P. Efavirenz Loaded Nanostructured Lipid Carriers for Efficient and Prolonged Viral Inhibition in HIV-Infected Macrophages. Pharm. Sci. 2020, 27, 418–432.
- Mahajan, K.; Rojekar, S.; Desai, D.; Kulkarni, S.; Bapat, G.; Zinjarde, S.; Vavia, P. Layer-by-Layer Assembled Nanostructured Lipid Carriers for CD-44 Receptor–Based Targeting in HIV-Infected Macrophages for Efficient HIV-1 Inhibition. AAPS PharmSciTech 2021, 22, 171.
- Rojekar, S.V.; Trimukhe, A.M.; Deshmukh, R.R.; Vavia, P.R. Novel pulsed oxygen plasma mediated surface hydrophılizatıon of ritonavır for the enhancement of wettability and solubility. J. Drug Deliv. Sci. Technol. 2021, 63, 102497.
- Rojekar, S.; Pai, R.; Abadi, L.F.; Mahajan, K.; Prajapati, M.K.; Kulkarni, S.; Vavia, P. Dual loaded nanostructured lipid carrier of nano-selenium and Etravirine as a potential anti-HIV therapy. Int. J. Pharm. 2021, 607, 120986.
- Rojekar, S.; Fotooh, L.; Pai, R.; Mahajan, K.; Kulkarni, S.; Vavia, P.R. Multi-organ Targeting of HIV-1 Viral Reservoirs with Etravirine Loaded Nanostructured Lipid Carrier: An In-vivo Proof of Concept. Eur. J. Pharm. Sci. 2021, 164, 105916.
- Brechtl, J.R.; Breitbart, W.; Galietta, M.; Krivo, S.; Rosenfeld, B. The Use of Highly Active Antiretroviral Therapy (HAART) in Patients with Advanced HIV Infection: Impact on Medical, Palliative Care, and Quality of Life Outcomes. J. Pain Symptom Manag. 2001, 21, 41–51.
- Simon, V.; Ho, D.D.; Karim, Q.A. HIV/AIDS epidemiology, pathogenesis, prevention, and treatment. Lancet 2006, 368, 489–504.
- Sharp, P.; Hahn, B.H. Origins of HIV and the AIDS Pandemic. Cold Spring Harb. Perspect. Med. 2011, 1, a006841.
- Nishioka, Y.; Yoshino, H. Lymphatic targeting with nanoparticulate system. Adv. Drug Deliv. Rev. 2001, 47, 55–64.
- Cavalu, S.; Banica., F.; Gruian, C.; Vanea, E.; Goller, G.; Simon, V. Microscopic and spectroscopic investigation of bioactive glasses for antibiotic controlled release. J. Mol. Struct. 2013, 1040, 47–52.
- Lee, E.S.; Na, K.; Bae, Y.H. Super pH-Sensitive Multifunctional Polymeric Micelle. Nano Lett. 2005, 5, 325–329.
- Kono, K. Thermosensitive polymer-modified liposomes. Adv. Drug Deliv. Rev. 2001, 53, 307–319.
- Kong, G.; Braun, R.D.; Dewhirst, M.W. Hyperthermia Enables Tumor-Specific Nanoparticle Delivery: Effect of Particle Size 1. Cancer Res. 2000, 60, 4440–4445.
- Chen, Q.; Krol, A.; Wright, A.; Nedham, D.; Dewhirst, M.W.; Yuan, F. Tumor microvascular permeability is a key determinant for antivascular effects of doxorubicin encapsulated in a temperature sensitive liposome. Int. J. Hyperth. 2008, 24, 475–482.
- Kim, E.; Yang, J.; Choi, J.; Suh, J.-S.; Huh, Y.-M.; Haam, S. Synthesis of gold nanorod-embedded polymeric nanoparticles by a nanoprecipitation method for use as photothermal agents. Nanotechnology 2009, 20, 365602.
- Kumar, P.; Ban, H.-S.; Kim, S.-S.; Wu, H.; Pearson, T.; Greiner, D.L.; Laouar, A.; Yao, J.; Haridas, V.; Habiro, K.; et al. T Cell-Specific siRNA Delivery Suppresses HIV-1 Infection in Humanized Mice. Cell 2008, 134, 577–586.
- Gagné, J.-F.; Désormeaux, A.; Perron, S.; Tremblay, M.J.; Bergeron, M.G. Targeted delivery of indinavir to HIV-1 primary reservoirs with immunoliposomes. Biochim. Et Biophys. Acta (BBA)—Biomembr. 2001, 1558, 198–210.
- Delcroix, M.; Riley, L.W. Cell-Penetrating Peptides for Antiviral Drug Development. Pharmaceuticals 2010, 3, 448–470.
- Azad, A.K.; Rajaram, M.V.S.; Schlesinger, L.S. Exploitation of the Macrophage Mannose Receptor (CD206) in Infectious Disease Diagnostics and Therapeutics. J. Cytol. Mol. Biol. 2014, 1, 1000003.
- Fraser, I.P.; Ezekowitz, R.A.B. Mannose Receptor and Phagocytosis. Adv. Cell. Mol. Biol. Membr. Organelles 1999, 5, 87–101.
- Shikuma, C.M.; Shiramizu, B. Mitochondrial toxicity associated with nucleoside reverse transcriptase inhibitor therapy. Curr. Infect. Dis. Rep. 2001, 3, 553–560.
- Bañó, M.; Morén, C.; Barroso, S.; Juárez, D.L.; Guitart-Mampel, M.; González-Casacuberta, I.; Canto-Santos, J.; Lozano, E.; León, A.; Pedrol, E.; et al. Mitochondrial Toxicogenomics for Antiretroviral Management: HIV Post-exposure Prophylaxis in Uninfected Patients. Front. Genet. 2020, 11, 497.
- Carr, A.; Miller, J.; Law, M.; Cooper, D.A. A syndrome of lipoatrophy, lactic acidaemia and liver dysfunction associated with HIV nucleoside analogue therapy: Contribution to protease inhibitor-related lipodystrophy syndrome. AIDS 2000, 14, F25–F32.
- Kim, J.; Song, S.Y.; Lee, S.G.; Choi, S.; Lee, Y.I.; Choi, J.Y.; Lee, J.H. Treatment of Human Immunodeficiency Virus-Associated Facial Lipoatrophy with Hyaluronic Acid Filler Mixed With Micronized Cross-Linked Acellular Dermal Matrix. J. Korean Med Sci. 2022, 37, e37.
- Baril, J.-G.; Junod, P.; LeBlanc, R.; Dion, H.; Therrien, R.; Laplante, F.; Falutz, J.; Côté, P.; Hébert, M.-N.; Lalonde, R.; et al. HIV-associated Lipodystrophy Syndrome: A Review of Clinical Aspects. Can. J. Infect. Dis. Med Microbiol. 2005, 16, 233–243.
- Guaraldi, G.; Stentarelli, C.; Zona, S.; Santoro, A. HIV-Associated Lipodystrophy: Impact of Antiretroviral Therapy. Drugs 2013, 73, 1431–1450.
- Kuo, H.-H.; Lichterfeld, M. Recent progress in understanding HIV reservoirs. Curr. Opin. HIV AIDS 2018, 13, 137–142.
- Liu, D.-Z.; Ander, B.P.; Xu, H.; Shen, Y.; Kaur, P.; Deng, W.; Sharp, F.R. Blood-brain barrier breakdown and repair by Src after thrombin-induced injury. Ann. Neurol. 2009, 67, 526–533.
- Teleanu, D.M.; Chircov, C.; Grumezescu, A.M.; Volceanov, A.; Teleanu, R.I. Blood-Brain Delivery Methods Using Nanotechnology. Pharmaceutics 2018, 10, 269.
- Banerjee, D.; Liu, A.P.; Voss, N.R.; Schmid, S.L.; Finn, M.G. Multivalent Display and Receptor-Mediated Endocytosis of Transferrin on Virus-Like Particles. ChemBioChem 2010, 11, 1273–1279.
- Jain, S.K.; Gupta, Y.; Jain, A.; Saxena, A.R.; Khare, P.; Jain, A. Mannosylated gelatin nanoparticles bearing an anti-HIV drug didanosine for site-specific delivery. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 41–48.
- Dutta, T.; Jain, N.K. Targeting potential and anti-HIV activity of lamivudine loaded mannosylated poly (propyleneimine) dendrimer. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2007, 1770, 681–686.
- Patel, B.K.; Parikh, R.H.; Patel, N. Targeted delivery of mannosylated-PLGA nanoparticles of antiretroviral drug to brain. Int. J. Nanomed. 2018, 13, 97–100.
- Kaur, A.; Jain, S.; Tiwary, A. Mannan-coated gelatin nanoparticles for sustained and targeted delivery of didanosine: In vitro and in vivo evaluation. Acta Pharm. 2008, 58, 61–74.
- Rao, K.S.; Reddy, M.K.; Horning, J.L.; Labhasetwar, V. TAT-conjugated nanoparticles for the CNS delivery of anti-HIV drugs. Biomaterials 2008, 29, 4429–4438.
- Kuo, Y.-C.; Wang, L.-J. Transferrin-grafted catanionic solid lipid nanoparticles for targeting delivery of saquinavir to the brain. J. Taiwan Inst. Chem. Eng. 2014, 45, 755–763.
- Lavigne, C.; Guedj, A.-S.; Kell, A.J.; Barnes, M.; Stals, S.; Gonçalves, D.; Girard, D. Preparation, characterization, and safety evaluation of poly (lactide-co-glycolide) nanoparticles for protein delivery into macrophages. Int. J. Nanomed. 2015, 10, 5965–5979.
- Shegokar, R. Preparation, Characterization and Cell Based Delivery of Stavudine Surface Modified Lipid Nanoparticles. J. Nanomed. Biother. Discov. 2012, 2.
- Yu, S.S.; Lau, C.M.; Barham, W.J.; Onishko, H.M.; Nelson, C.E.; Li, H.; Smith, C.A.; Yull, F.E.; Duvall, C.L.; Giorgio, T.D. Macrophage-Specific RNA Interference Targeting via “Click”, Mannosylated Polymeric Micelles. Mol. Pharm. 2013, 10, 975–987.
- Kedzierska, K. The Role of Monocytes and Macrophages in the Pathogenesis of HIV-1 Infection. Curr. Med. Chem. 2002, 9, 1893–1903.
- Jamari, J.; Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Prakoso, A.T.; Basri, H.; van der Heide, E. Computational Contact Pressure Prediction of CoCrMo, SS 316L and Ti6Al4V Femoral Head against UHMWPE Acetabular Cup under Gait Cycle. J. Funct. Biomater. 2022, 13, 64.
- Garg, M.; Dutta, T.; Jain, N.K. Reduced hepatic toxicity, enhanced cellular uptake and altered pharmacokinetics of stavudine loaded galactosylated liposomes. Eur. J. Pharm. Biopharm. 2007, 67, 76–85.
- Cao, S.; Woodrow, K.A. Nanotechnology Approaches to Eradicating HIV Reservoirs. Eur. J. Pharm. Biopharm. 2019, 138, 48–63.
- Jindal, A.; Bachhav, S.; Devarajan, P.V. In situ hybrid nano drug delivery system (IHN-DDS) of antiretroviral drug for simultaneous targeting to multiple viral reservoirs: An in vivo proof of concept. Int. J. Pharm. 2017, 521, 196–203.
- Shibata, A.; McMullen, E.; Pham, A.; Belshan, M.; Sanford, B.; Zhou, Y.; Goede, M.; Date, A.; Destache, C.J. Polymeric Nanoparticles Containing Combination Antiretroviral Drugs for HIV Type 1 Treatment. AIDS Res. Hum. Retroviruses 2013, 29, 746–754.
- Liu, C.; Zhou, Q.; Li, Y.; Garner, L.V.; Watkins, S.P.; Carter, L.J.; Smoot, J.; Gregg, A.C.; Daniels, A.D.; Jervey, S.; et al. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. ACS Cent. Sci. 2020, 6, 315–331.
- Malik, T.; Chauhan, G.; Rath, G.; Kesarkar, R.N.; Chowdhary, A.S.; Goyal, A.K. Efaverinz and nano-gold-loaded mannosylated niosomes: A host cell-targeted topical HIV-1 prophylaxis via thermogel system. Artif. Cells Nanomed. Biotechnol. 2017, 46, 79–90.
- Vieira, A.C.; Magalhães, J.; Rocha, S.; Cardoso, M.S.; Santos, S.G.; Borges, M.; Pinheiro, M.; Reis, S. Targeted macrophages delivery of rifampicin-loaded lipid nanoparticles to improve tuberculosis treatment. Nanomedicine 2017, 12, 2721–2736.
- Makabi-Panzu, B.; Lessard, C.; Beauchamp, D.; Désormeaux, A.; Poulin, L.; Tremblay, M.; Bergeron, M.G. Uptake and Binding of Liposomal 2’,3’-Dideoxycytidine by RAW 264.7 Cells: A Three-Step Process. J. Acquir. Immune Defic. Syndr. Hum. Retrovirology 1995, 8, 227–235.
- Hillaireau, H.; Couvreur, P. Nanocarriers’ entry into the cell: Relevance to drug delivery. Cell. Mol. Life Sci. 2009, 66, 2873–2896.
- Kaur, C.D.; Nahar, M.; Jain, N.K. Lymphatic targeting of zidovudine using surface-engineered liposomes. J. Drug Target. 2008, 16, 798–805.
- Kulkarni, S.A.; Feng, S.-S. Effects of Particle Size and Surface Modification on Cellular Uptake and Biodistribution of Polymeric Nanoparticles for Drug Delivery. Pharm. Res. 2013, 30, 2512–2522.
- Klatzmann, D.; Barré-Sinoussi, F.; Nugeyre, M.T.; Danguet, C.; Vilmer, E.; Griscelli, C.; Brun-Veziret, F.; Rouzioux, C.; Gluckman, J.C.; Chermann, J.-C.; et al. Selective Tropism of Lymphadenopathy Associated Virus (LAV) for Helper-Inducer T Lymphocytes. Science 1984, 225, 59–63.
- Hajjar, A.M.; Lewis, P.F.; Endeshaw, Y.; Ndinya-Achola, J.; Kreiss, J.K.; Overbaugh, J. Efficient Isolation of Human Immunodeficiency Virus Type 1 RNA from Cervical Swabs. J. Clin. Microbiol. 1998, 36, 2349–2352.
- Jain, A.; Agarwal, A.; Majumder, S.; Lariya, N.; Khaya, A.; Agrawal, H.; Majumdar, S.; Agrawal, G.P. Mannosylated solid lipid nanoparticles as vectors for site-specific delivery of an anti-cancer drug. J. Control. Release 2010, 148, 359–367.
- Shahiwala, A.; Amiji, M.M. Nanotechnology-based delivery systems in HIV/AIDS therapy. Futur. HIV Ther. 2007, 1, 49–59.
- Edagwa, B.J.; Zhou, T.; McMillan, J.M.; Liu, X.-M.; E Gendelman, H. Development of HIV Reservoir Targeted Long Acting Nanoformulated Antiretroviral Therapies. Curr. Med. Chem. 2014, 21, 4186–4198.
- Eisele, E.; Siliciano, R.F. Redefining the Viral Reservoirs that Prevent HIV-1 Eradication. Immunity 2012, 37, 377–388.
- Trimukhe, A.; Rojekar, S.; Vavia, P.R.; Deshmukh, R. Pulsed plasma surface modified omeprazole microparticles for delayed release application. J. Drug Deliv. Sci. Technol. 2021, 66, 102905.
- Nie, S. Understanding and Overcoming Major Barriers in Cancer Nanomedicine. Opsonization Phagocytosis 2010, 5, 523–528.
- Campagne, M.V.L.; Wiesmann, C.; Brown, E.J. Macrophage complement receptors and pathogen clearance. Cell. Microbiol. 2007, 9, 2095–2102.
- Litzinger, D.C.; Buiting, A.M.; van Rooijen, N.; Huang, L. Effect of liposome size on the circulation time and intraorgan distribution of amphipathic poly(ethylene glycol)-containing liposomes. Biochim. Biophys. Acta (BBA)—Biomembr. 1994, 1190, 99–107.
- Saste, V.S.; Kale, S.S.; Sapate, M.K.; Baviskar, D.T. Modern Aspects for Antiretroviral Treatment. Int. J. Pharm. Sci. Rev. Res. 2011, 9, 18–24.
- Raina, H.; Kaur, S.; Jindal, A.B. Development of efavirenz loaded solid lipid nanoparticles: Risk assessment, quality-by-design (QbD) based optimisation and physicochemical characterisation. J. Drug Deliv. Sci. Technol. 2017, 39, 180–191.
- Alukda, D.; Sturgis, T.; Youan, B.C. Formulation of tenofovir-loaded functionalized solid lipid nanoparticles intended for HIV prevention. J. Pharm. Sci. 2011, 100, 3345–3356.
- Hari, B.V.; Narayanan, N.; Dhevendaran, K.; Ramyadevi, D. Engineered nanoparticles of Efavirenz using methacrylate co-polymer (Eudragit-E100) and its biological effects in-vivo. Mater. Sci. Eng. C 2016, 67, 522–532.
- Iannazzo, D.; Pistone, A.; Romeo, R.; Giofrè, S.V. Nanotechnology Approaches for Antiretroviral Drugs Delivery. J. AIDS HIV Infect. 2015, 1, 1–13.
- Kotta, S.; Khan, A.W.; Ansari, S.H.; Sharma, R.K.; Ali, J. Anti HIV nanoemulsion formulation: Optimization and in vitro–in vivo evaluation. Int. J. Pharm. 2014, 462, 129–134.
- Garg, B.; Beg, S.; Kumar, R.; Katare, O.; Singh, B. Nanostructured lipidic carriers of lopinavir for effective management of HIV-associated neurocognitive disorder. J. Drug Deliv. Sci. Technol. 2019, 53, 101220.
- Chakraborty, T.; Das, M.K.; Dutta, L.; Mukherjee, B.; Das, S.; Sarma, A. Successful Delivery of Zidovudine-Loaded Docosanol Nanostructured Lipid Carriers (Docosanol NLCs) into Rat Brain. In Surface Modification of Nanoparticles for Targeted Drug Delivery; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 245–276.
- Dusserre, N.; Lessard, C.; Paquette, N.; Perron, S.; Poulin, L.; Tremblay, M.; Beauchamp, D.; Désormeaux, A.; Bergeron, M.G. Encapsulation of foscarnet in liposomes modifies drug intracellular accumulation, in vitro anti-HIV-1 activity, tissue distribution, and pharmacokinetics. AIDS 1995, 9, 833–842.
- Freitas, R.A. Pharmacytes: An Ideal Vehicle for Targeted Drug Delivery. J. Nanosci. Nanotechnol. 2006, 6, 2769–2775.
- Houacine, C.; Adams, D.; Singh, K. Impact of liquid lipid on development and stability of trimyristin nanostructured lipid carriers for oral delivery of resveratrol. J. Mol. Liq. 2020, 316, 113734.
- Désormeaux, A.; Harvie, P.; Perron, S.; Makabi-Panzu, B.; Beauchamp, D.; Tremblay, M.; Poulin, L.; Bergeron, M.G. Antiviral efficacy, intracellular uptake and pharmacokinetics of free and liposome-encapsulated 2’,3’-dideoxyinosine. AIDS 1994, 8, 1545–1554.
- Purvin, S.; Vuddanda, P.R.; Singh, S.K.; Jain, A. Pharmacokinetic and Tissue Distribution Study of Solid Lipid Nanoparticles of Zidovudine in Rats. J. Nanotechnol. 2014, 2014, 1–7.
- Pharmacokinetic and Tissue Distribution of Zidovudine in Rats Following Intravenous Administration of Zidovudine Myristate Loaded Liposomes. Available online: https://www.researchgate.net/publication/7448025_Pharmacokinetic_and_tissue_distribution_of_zidovudine_in_rats_following_intravenous_administration_of_zidovudine_myristate_loaded_liposomes (accessed on 22 March 2021).
- Destache, C.J.; Belgum, T.; Goede, M.; Shibata, A.; Belshan, M.A. Antiretroviral release from poly (DL-lactide-co-glycolide) nanoparticles in mice. J. Antimicrob. Chemother. 2010, 65, 2183–2187.
- Publications. Available online: https://universe.bits-pilani.ac.in/Hyderabad/punnaraoravi/Publications (accessed on 22 March 2021).
- Kawai, T.; Akira, S. Toll-like Receptor and RIG-1-like Receptor Signaling. Ann. N. Y. Acad. Sci. 2008, 1143, 1–20.
- Davidson, I.; Beardsell, H.; Smith, B.; Mandalia, S.; Bower, M.; Gazzard, B.; Nelson, M.; Stebbing, J. The frequency and reasons for antiretroviral switching with specific antiretroviral associations: The SWITCH study. Antivir. Res. 2010, 86, 227–229.
- Gaur, P.K.; Mishra, S.; Bajpai, M.; Mishra, A. Enhanced Oral Bioavailability of Efavirenz by Solid Lipid Nanoparticles: In Vitro Drug Release and Pharmacokinetics Studies. BioMed Res. Int. 2014, 2014, 1–9.
- Zardini, A.A.; Mohebbi, M.; Farhoosh, R.; Bolurian, S. Production and characterization of nanostructured lipid carriers and solid lipid nanoparticles containing lycopene for food fortification. J. Food Sci. Technol. 2017, 55, 287–298.
- Talegaonkar, S.; Bhattacharyya, A. Potential of Lipid Nanoparticles (SLNs and NLCs) in Enhancing Oral Bioavailability of Drugs with Poor Intestinal Permeability. AAPS PharmSciTech 2019, 20, 121.
- Desai, J.; Thakkar, H. Darunavir-Loaded Lipid Nanoparticles for Targeting to HIV Reservoirs. AAPS PharmSciTech 2017, 19, 648–660.
- Bhalekar, M.R.; Upadhaya, P.G.; Madgulkar, A.R.; Kshirsagar, S.J.; Dube, A.; Bartakke, U.S. In-vivo bioavailability and lymphatic uptake evaluation of lipid nanoparticulates of darunavir. Drug Deliv. 2015, 23, 2581–2586.
- Stavudine Entrapped Lipid Nanoparticles for Targeting Lymphatic HIV Reservoirs—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/21612153/ (accessed on 30 August 2020).
- Kuo, Y.-C.; Chung, J.-F. Physicochemical properties of nevirapine-loaded solid lipid nanoparticles and nanostructured lipid carriers. Colloids Surf. B Biointerfaces 2011, 83, 299–306.
- Khan, A.A.; Mudassir, J.; Akhtar, S.; Murugaiyah, V.; Darwis, Y. Freeze-Dried Lopinavir-Loaded Nanostructured Lipid Carriers for Enhanced Cellular Uptake and Bioavailability: Statistical Optimization, in Vitro and in Vivo Evaluations. Pharmaceutics 2019, 11, 97.
- Endsley, A.N.; Ho, R.J. Enhanced Anti-HIV Efficacy of Indinavir After Inclusion in CD4-Targeted Lipid Nanoparticles. JAIDS J. Acquir. Immune Defic. Syndr. 2012, 61, 417–424.
- Makwana, V.; Jain, R.; Patel, K.; Nivsarkar, M.; Joshi, A. Solid lipid nanoparticles (SLN) of Efavirenz as lymph targeting drug delivery system: Elucidation of mechanism of uptake using chylomicron flow blocking approach. Int. J. Pharm. 2015, 495, 439–446.
- Park, K.S.; Bazzill, J.D.; Son, S.; Nam, J.; Shin, S.W.; Ochyl, L.J.; Stuckey, J.A.; Meagher, J.L.; Chang, L.; Song, J.; et al. Lipid-based vaccine nanoparticles for induction of humoral immune responses against HIV-1 and SARS-CoV-2. J. Control Release 2020, 330, 529–539.
- Saunders, K.O.; Pardi, N.; Parks, R.; Santra, S.; Mu, Z.; Sutherland, L.; Scearce, R.; Barr, M.; Eaton, A.; Hernandez, G.; et al. Lipid nanoparticle encapsulated nucleoside-modified mRNA vaccines elicit polyfunctional HIV-1 antibodies comparable to proteins in nonhuman primates. npj Vaccines 2021, 6, 50.
- Bae, M.; Kim, H. The Role of Vitamin C, Vitamin D, and Selenium in Immune System against COVID-19. Molecules 2020, 25, 5346.
- Cathcart, R.F. Vitamin C in the treatment of acquired immune deficiency syndrome (AIDS). Med. Hypotheses 1984, 14, 423–433.
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391.
- Berretta, M.; Quagliariello, V.; Maurea, N.; Di Francia, R.; Sharifi, S.; Facchini, G.; Rinaldi, L.; Piezzo, M.; Manuela, C.; Nunnari, G.; et al. Multiple Effects of Ascorbic Acid against Chronic Diseases: Updated Evidence from Preclinical and Clinical Studies. Antioxidants 2020, 9, 1182.
- Mallipeddi, R.; Rohan, L.C. Progress in antiretroviral drug delivery using nanotechnology. Int. J. Nanomed. 2010, 5, 533–547.
- Kumar, L.; Verma, S.; Prasad, D.N.; Bhardwaj, A.; Vaidya, B.; Jain, A.K. Nanotechnology: A magic bullet for HIV AIDS treatment. Artif. Cells Nanomed. Biotechnol. 2014, 43, 71–86.
- Date, A.A.; Destache, C.J. A review of nanotechnological approaches for the prophylaxis of HIV/AIDS. Biomaterials 2013, 34, 6202–6228.
- Gupta, U.; Jain, N.K. Non-polymeric nano-carriers in HIV/AIDS drug delivery and targeting. Adv. Drug Deliv. Rev. 2010, 62, 478–490.
