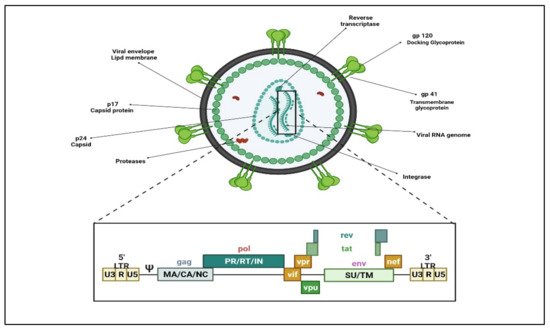
HIV (HIV-1 and HIV-2) belongs to the retroviruses family within the genus lentivirus. As with all retroviruses, the genome in the virus particle is diploid, comprising of two single-stranded RNA molecules. On infection, the viral enzyme reverse transcriptase catalyzes the synthesis of a haploid, double-stranded DNA provirus that becomes inserted into the chromosomal DNA of a host cell and tricks the host biosynthetic machinery for its protein synthesis. The genome contains 10 open reading frames, encoding several crucial viral gene products that provide structural integrity to the virion and enzymatic functions for viral replication or regulating viral gene expression. (Figure 1) Reverse transcriptase (RT), the viral polymerase responsible for retro-transcribing viral RNA to double-stranded DNA, lacks proofreading, thus making the process of viral genetic replication susceptible to errors. Viral subtypes are formed when such errors steadily accumulate, gradually leading to the evolution of viruses that could vary significantly from one another at the genetic level.
There are three main groups within HIV-1, i.e., M (major), N, and O (outliner). M comprises most of the HIV-1 strains, which comprise at least 10 to 12 genetically separate sub-types labeled A through J. Additionally, group O consists of a separate group of viruses recognized primarily in Cameroon. A small number of strains genetically different from the above two groups have primarily been limited to Cameroon and Gabon. These are located in group N. Nevertheless, the divergence of sub-types is a dynamic, continuing process, and many viral sub-types are still being identified. Numerous strains persist uncategorized since they fail to segregate with any known groups.
2. Nanopharmaceuticals; Novel Directions on HIV/AIDS Treatment Approaches
Novel ART approaches and nanopharmaceuticals have displayed promising results to a certain extent in HIV/AIDS therapy. Nanomedicine is a field of medicine that employs nanotechnology for the prevention and treatment of diseases utilizing NPs, such as biocompatible NPs [
99] and nanorobots [
100] for numerous applications comprising diagnosis [
101], delivery [
102], sensory [
103], or the actuation purposes in a living organism [
104]. Nanomedicine provides novel approaches to preventing viral infection, growth, and transmission. Many inorganic and metal NPs (i.e., gold, silver, and silica nanoparticles) have been intensively studied for application in imaging, bioassays, and therapeutics [
105,
106,
107,
108,
109]. Despite significant advances in HIV/AIDS treatment, there are many lacunas in the HIV/AIDS treatments addressed by nanoart, which target different stages of the HIV lifecycle as per the drug/s mechanism of action [
110] (
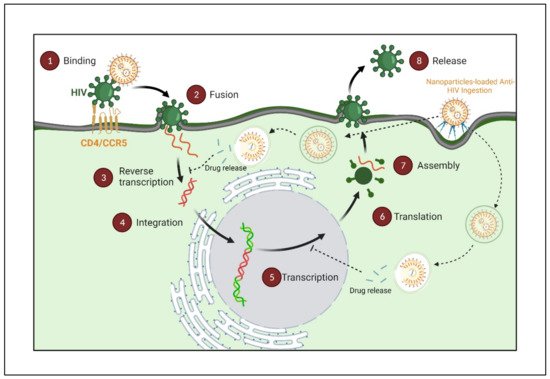
Figure 5).
Figure 5. HIV sites for therapeutic intervention using nanopharmaceuticals. (Created with Biorender.com (access on 19 June 2022)).
3. Nanoparticles Transport Approaches
Most nanoparticulate drug delivery systems need to cross the cell membrane and deliver the cargo (ART) to elicit an antiviral response. Hence it is imperative to familiarize oneself with the nanoparticle transport approaches. There are multiple ways for nanoparticles to cross the cell membranes, primarily through paracellular or transcellular pathways (
Figure 6). The majority, however, reach the target cells via a substrate-specific process known as the endogenous transporter or carrier-mediated route determined by the concentration gradient of the substrates with the help of appropriate transporters [
96,
111,
112].
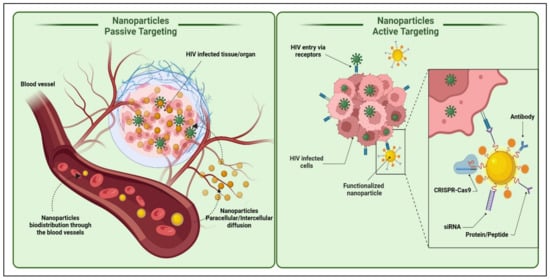
Figure 6. Active and passive uptake of nanoparticles to the HIV-infected tissues/organs. The active targeting strategy can include nanoparticle functionalization directly or indirectly with various molecules; such as drugs, nucleic acids (DNA or RNA), proteins or peptides, antibodies, etc., for ideal biological activities and diverse medical applications. (Created with BioRender.com (access on 19 June 2022)).
3.1. Active Transport
Active targeting involves the specific modification of drug/drug-loaded nanocarriers with site-specific ligands or “active” agents, which have a discriminating or specific affinity for distinguishing and interacting with a specific cell, tissue, or organ in the body based on its protein expression profile [
113]. An active targeting approach significantly increases the possibility of the drug target cell’s interaction while sparing healthy cells. The active targeting of particulate carriers can be further classified as;
Stimuli-Responsive Nanocarriers
Stimuli-sensitive nanocarriers are based on internal and external stimuli. The internal stimulus includes tumor microenvironment pH and temperature, while the external stimulus includes hyperthermia magnetic field or ultrasound energy [
97,
98,
99,
114,
115,
116].
Antibody Targeted Nanocarriers
An antibody having a specific affinity towards an antigen found on the target cell’s surface could be anchored on the nanocarriers’ surface to increase their targeting efficiency. Earlier, this approach was widely explored in cancer therapy, but currently, it has become a key interest in HIV/AIDS therapy. HIV-infected cells express various molecules, such as gp120, the HLA-DR determinant of MHC-II, and CD receptors on their surface, which may be targeted by whole antibodies or fragments of an antibody. Immunoliposomes are widely explored nanocarriers amongst various nanocarriers. Cells, such as follicular dendritic cells, B cells, and macrophages can express the HLA-DR determinant of MHC-II and be theoretically targeted by anti HLA-DR monoclonal antibodies. Immunoliposomes anchored with anti-HLA-DR monoclonal antibodies revealed enhanced accumulation of Indinavir in the lymph nodes, with greater than a 126-fold area-under-the-curve than the free drugs in mice. Kumar et al. demonstrated the effective suppression of HIV infection in humanized mice by targeted siRNA delivery to T cells using an antibody (scFvCD7) specific to the CD7 receptor on the T cells [
100,
101,
102].
Receptor-Mediated Endocytosis (RME)
Target cells express various receptors on their surface to enable the internalization of drug-loaded cargoes into the cell and their degradation. The binding of ligand conjugated nanocarriers to receptors present on the cell surface generates a sequence of cellular actions that result in their internalization within the cell. Phagocytic processes are faster than RME, with the ligand playing an essential role in RME. The common RME mechanisms are micropinocytosis, clathrin-dependent endocytosis, caveolae-mediated endocytosis, and clathrin-independent endocytosis. Further, the uptake mechanism is often dependent on the nature of the ligand [
96]. Macrophages are the primary differentiating cells of the mononuclear phagocyte system and are also responsible for disseminating the infection throughout the body, as mentioned elsewhere. Macrophages residing in the above-mentioned organs serve as a potential reservoir for HIV [
97,
98,
113]. Targeting anti-HIV1 drugs to these macrophages residing in multiple HIV reservoirs would significantly benefit the therapy because many anti-HIV1 drugs administered via the conventional routes fail to penetrate these sites optimally.
The d-Mannose Receptor Targeting
The d-mannose receptor (MR, CD206, or MRC1) is a transmembrane glycoprotein classified under the C-type lectin family and present on most macrophages’ surfaces. Its extracellular regions consist of an N-terminal cysteine-rich (CR) domain, which has an affinity to glycoproteins bearing sulfated sugars glycoproteins terminating in 4-SO4GalNAc, a fibronectin II (FNII) domain, and eight carbohydrate recognition domains (CRDs) that bind sugars, such as d-mannose and fucose, with high-affinity. Regardless of the continued development of drug delivery technologies, the effective targeting of drugs to macrophages to treat the underlying diseases remains proven [
103,
121]. Mannosylation is currently the best strategy to develop nanomedicines that target d-mannose receptors, which are highly expressed in cells of the immune system [
109,
133,
134,
135]. Based on the growing literature, nanocarrier mannosylation will increase uptake by macrophages to provide clinically relevant concentrations in target tissues or organs.
Moreover, improved uptake is projected to require lower doses of the agents sufficient for therapeutic effects, thus providing reduced toxicity. Researchers have formulated mannosylated polymeric micelles for high efficient delivery of siRNA into macrophages [
136]. Bhavin et al. have developed mannosylated PLGA nanoparticles that improve brain bioavailability [
133,
137]. Moreover, different novel drug delivery approaches in combination with mannosylation for the improvement in selective macrophage uptake, such as polymeric nanoparticle [
133,
138], polysaccharide-based vaccine [
139], liposome [
121], niosomes [
140], NLC [
134], dendrimer [
135], solid lipid nanoparticles (SLN) [
141], chitosan nanoparticles [
62,
142], and gelatin nanoparticles [
128] have been evaluated.
3.2. Passive Targeting
Passive targeting involves accumulating the drug-carrier system at a site due to physicochemical or pharmacological factors [
1,
42,
46]. Nanoparticle accumulation is observed in the liver due to the large fenestrations. The nanocarriers are readily taken up by the monocyte phagocytic system (MPS) cells or reticuloendothelial system (RES) through phagocytosis. RES consists of fixed macrophage cells in organs, mainly the liver (Kupffer cells) and spleen, lung, kidney, bone marrow, circulating monocytes, macrophages, and polymorph nuclear leukocytes cells [
143,
144]. These RES cells cannot identify the particulate carriers themselves but recognize specialized opsonin proteins deposited on the particle surface, followed by MPS uptake [
145].
Endocytosis
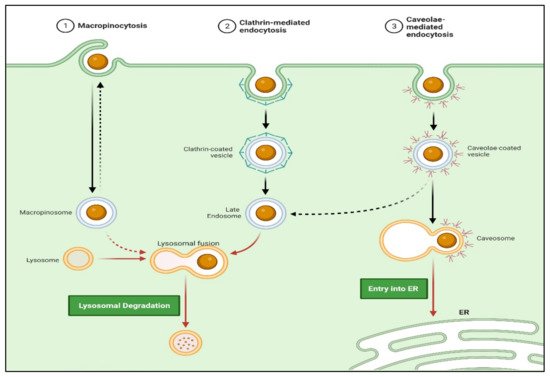
Approaches involving passive targeting can result in the accumulation of higher concentrations of drugs at the target sites. This local gradient difference may allow the drug penetration by passive diffusion. Moreover, trafficking via non-receptor-mediated endocytosis (i.e., macropinocytosis) may enhance cellular drug uptake. Actively targeted drug trafficking can be possible via receptor-mediated endocytosis when the periphery of nanocarriers is tagged with ligand molecules matching the specific cell receptor (
Figure 7) [
112].
Figure 7. Schematic representation of nanocarrier internalization via various endocytosis mechanisms. (Created with BioRender.com (accessed on 19 June 2022)).
Phagocytosis
It is a process by which cells, generally macrophages, engulf solid particles. The first step of phagocytosis is opsonization, in which opsonin (antibody or complement molecules) cover solid particles [
146]. Phagocytic cells express Fc and CR1 receptors that bind opsonin molecules and antibodies and complement C3b. Following ingestion, solid particles become trapped in phagocytic vesicles (phagosomes), which fuse with intracellular organelles containing digestive proteins and an acidic internal pH and mature into phagolysosomes that degrade the internalized nano drug delivery system. The nano drug delivery system is then eliminated by exocytosis after degradation or sequestered in residual cells’ bodies if it cannot be digested. Contacts between the nano drug delivery system and macrophages occur via the recognition of opsonin on the nano drug delivery system surface or through interactions with scavenger receptors on macrophages. This fact can target macrophages passively, lymph nodes, and the spleen to treat infections that affect RES (HIV/AIDS) [
109,
147,
148]. Drug or drug carrier nanosystems can be passively targeted by manipulating their physicochemical factors, such as size, shape, surface charge, and surface hydrophobicity [
46,
130,
149].
4. Factors Impacting the Functionalities of Nanocarrier Targeted Delivery
4.1. Particle Size
Particle size affects the bioavailability and circulation time of the nanocarriers [
134]. It also decides the mechanism through which it moves in the cell and its localization. The particle size of nanocarriers is suitable for passive targeting of various HIV reservoir sites. Nanoparticles of particle size >200 nm are opsonized, phagocytosed, and taken up by the macrophages of the RES organs, major HIV reservoir sites, while <200 nm escape phagocytosis and localize in remote reservoirs, such as bone marrow, brain, and gonads, in high concentrations [
43].
HIV infection is an intracellular infection of macrophages localized primarily in the reticuloendothelial system (RES) organs, including the liver, spleen, lung, lymph node, genitals, lymphocytes, and brain [
39]. Treating HIV infection with conventional therapies is unmanageable because of their major prevailing limitations, such as poor efficacy and drug resistance [
150]. The drug did not reach the infected site in conventional therapies due to several HIV reservoir barriers [
39,
96]. Moreover, in the case of nanomedicine, it could passively or actively target these viral reservoir sites and eradicate the HIV infection [
42,
131,
151]. Scientists have been studied the numerous nanomedicine for the improvement of anti-HIV therapies, such as liposome [
54,
152]; solid lipid nanoparticle [
147,
153]; polymeric nanoparticle [
148,
149]; and nanoemulsion [
125,
154], and nanostructured lipid carriers (NLCs) [
143,
155].
4.2. Particle Shape
More recently, the effect of particle shape on cell uptake and biodistribution has been recognized. In one study, Mitragotri and Champion reported the surprising finding that particle size and particle shape also affect phagocytosis [
151,
156]. A macrophage internalized ellipse pointed end in a few minutes, while the same ellipse has not internalized via the flat region even over 12 h. Spherical nanoparticles were internalized through any point of attachment because of their symmetry. The asymmetric lipomer of doxycycline hydrochloride and amphotericin B revealed enhanced splenic uptake following intravenous administration [
43,
157].
4.3. Surface Charge
Surface charge and functional groups present on the surface of particulate carrier influence interaction with the cells and further traverse across the negatively charged cell membrane. Positively charged nanoparticles demonstrated higher phagocytic uptake than neutral hydrophilic or negatively charged nanoformulations [
158]. For instance, polystyrene nanoparticles with a primary amine group at the surface underwent significantly more phagocytosis than nanoparticles with sulfate, hydroxyl, and carboxyl groups. The extended blood circulation half-life of negatively charged particulate carriers could be due to the reduced adsorption of opsonin [
159]. Further, negatively charged nanoparticles could effectively bound to cationic sites on the macrophages at the scavenger receptors, enabling their uptake by RES [
160]. The cellular entry of antiretroviral agents that are negatively charged, such as phosphorylated nucleotide analogs and nucleic acids, get faster entry into the cells. The siRNA dendriplexes delivered to human astrocytes decreased the replication of HIV-1 due to higher intracellular concentration [
161].
4.4. Surface Hydrophobicity
The systemic circulation of particulate carriers is strongly influenced by surface hydrophobicity. Particles with hydrophobic surfaces are coated by the complement proteins, albumin, and immunoglobulins and further rapidly cleared by RES (reticuloendothelial system) from the circulation than those with a hydrophilic surface. Surface hydrophobicity thus impacts opsonization, phagocytosis, and the biodistribution of nanoparticles [
162,
163].
5. Liposomes-Based Delivery Systems for Anti-HIV Therapeutics
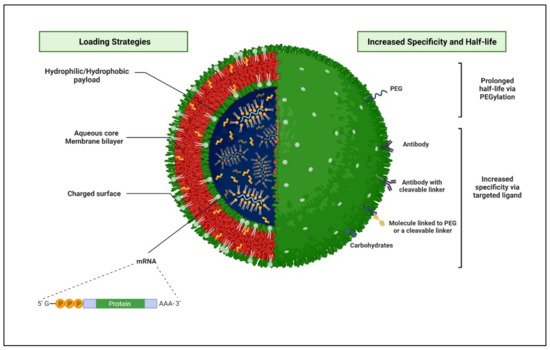
Liposomes are small artificial spherical vesicles constituted by one or more phospholipid bilayers with the polar groups of phospholipids oriented to the inner and outer aqueous phase. Such a structure explains the high propensity of liposomes to be encapsulated with hydrophilic, hydrophobic, and amphiphilic drugs within the inner aqueous compartment, the lipid bilayers, and at their interfaces, respectively (Figure 8).
Figure 8. Schematic 3D representation of a structural arrangement of a liposome and various loading strategies, which are leading to enhancing the specificity and half-life of the nanocarrier at the targeted HIV-infected sites. (Created with BioRender.com (accessed on 19 June 2022).
In this review, NLCs were selected over other nanoparticle constructs because they offer numerous advantages, including controlled release of drug and targeting, improved drug stability, and the capability to incorporate lipophilic and hydrophilic drugs biocompatibility [
131,
157,
164,
165]. The latter advantage is that NLCs are made of whichever physiological lipids or lipids are usually used as pharmaceutical excipients [
166,
167]. The nanocarrier-mediated targeted delivery of anti-retrovirals to HIV reservoirs has revealed great potential [
1,
46,
131,
151]. The darunavir-loaded lipid nanoparticle biodistribution study showed higher uptake in the spleen and brain HIV reservoirs [
168,
169]. Few reports suggest that stavudine-loaded lipid nanocarriers were utilized to deliver effectively to cellular and anatomical HIV reservoirs, increasing therapeutic safety [
121,
170]. Other studies have reported that nevirapine-loaded SLN and NLCs are used for effective and targeted delivery to HIV reservoirs [
167]. In vivo pharmacokinetics assessment of lopinavir-loaded with NLCs in rats has shown a 2.8-fold enhancement in brain uptake [
155,
167].
Zidovudine (AZT)-loaded docosanol NLCs were confined in the brain in a sustained-release method for an extended period [
143]. An AZT-loaded liposome has also shown an improvement in the uptake of lymphoid organs [
124]. Significant lymphoid tissue drug localization was found with the indinavir-associated lipid nanoparticles (LNPs) [
171]. SLN of efavirenz has shown a substantial amount of efavirenz in the spleen, a major lymphatic organ for better managing HIV [
172]. Tenofovir-loaded SLNs improved the cellular uptake of hydrophobic microbicides, which prevent the virus during the infection process and reduce the possible infections [
147].
Moreover, currently, the HIV vaccine is designed using a lipid-based nanoparticle vaccine platform (NVP) that presents HIV-1 viral proteins in a conformational manner for the induction of antigen-specific antibody responses. This type of vaccine can co-deliver protein antigens and adjuvants, which can boost immunogenicity by promoting their distribution to antigen-presenting cells [
164]. The current health crisis of coronavirus disease 2019 (COVID-19) highlights the safety of these approaches for generating potent and protective immune responses. In preclinical investigations, nucleoside-modified mRNAs formulated in lipid nanoparticles (mRNA-LNP) have proven to be a strong strategy of vaccination against infectious diseases, and are now being explored in people for SARS-CoV-2 [
165].
Liposomes-Based Delivery Systems of Ascorbic Acid to Increase the Bioavailability of ARTs
Vitamin C (ascorbic acid) is an essential water-soluble nutrient that functions as a cofactor for numerous enzymatic reactions. Vitamin C also serves as an antioxidant, anti-inflammatory, immunomodulatory, anti-viral, and anti-thrombotic agent and can potentially be used as a therapeutic or prophylactic agent [
190]. Preliminary clinical evidence showed that massive doses of ascorbic acid (50–200 g per day) can suppress the symptoms of the HIV disease and can markedly reduce the tendency for secondary infections [
191]. Oxidative stress influences viral replication, inflammatory responses, and immune cell proliferation, all of which contribute to the pathogenesis of HIV/AIDS. Hence, vitamin C can be used to reduce the damage caused by oxidative stress. Given the well-defined involvement of several lipids in the physiology of phagocytosis, the use of bioactive lipid nanoparticles to alter the phagosome maturation process has been proposed as a way to boost the efficacy of innate immunity mechanisms [
192].
Many experimental findings suggested that a liposome-based delivery system of ascorbic acid may be potentially beneficial to reduce oxidative stress and prevent several HIV chronic conditions and immune system activation. This strategy might help the oral administration of the ARTs [
193]. However, more research and trials are needed to determine the effects of vitamin C on HIV disease progression and prognosis.
5. Nanotechnological Advantages for Effective Anti-HIV Therapy
Nanotechnology-based nanosize drug-loaded carrier design presents manifold advantages, including targeting different anatomical and cellular viral reservoirs, thereby completely eradicating the virus from the reservoir sites. Targeted drug delivery at the site of action improves the drug’s efficacy and reduces the off-target effect. Nanocarriers deliver drugs in a controlled manner, increasing residence time at the target sites, increasing bioavailability, and improving the quality of life of HIV patients [
125,
194].
More importantly, a salient feature of nanotechnology that can be exploited for anti-HIV therapy is altered
in vivo biodistribution [
1,
195,
196,
197]. Although conventional drug distribution in the body is based on drug physicochemical properties, the drug properties are overshadowed to achieve carrier-mediated distribution when loaded in nanocarriers [
198,
199]. The physicochemical properties of the nanocarriers then become the rate-determining factor for the distribution in the body [
200,
201,
202,
203,
204,
205]. Therefore, altering the same could provide a promising approach to targeting HIV reservoir sites for effective anti-HIV therapy. Targeted drug delivery could be achieved via passive or active targeting [
57,
206,
207].