|
Type of Nerves
|
Name of Molecules
|
Target Cells
|
Effect
|
References
|
|
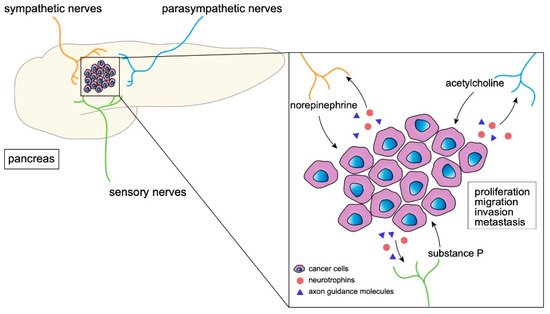
sympathetic nerves
|
norepinephrine, epinephrine
|
cancer cells
|
tumor progression
|
[29,35,39,51,52,53,54]
|
| |
|
immune cells
|
immune suppression
|
[29,55,56,57]
|
| |
|
endothelial cells
|
angiogenesis
|
[13,14,58,59]
|
| |
GABA
|
cancer cells
|
tumor suppression
|
[60]
|
| |
|
|
tumor progression
|
[61]
|
| |
dopamine
|
endothelial cells
|
suppression of angiogenesis
|
[62,63,64,65]
|
| |
NGF, BDNF
|
cancer cells
|
tumor progression
|
[66,67]
|
| |
GFRα1
|
cancer cells
|
tumor progression
|
[68,69]
|
| |
CX3CL1
|
cancer cells
|
tumor progression
|
[70]
|
|
parasympathetic nerves
|
acetylcholine
|
cancer cells
|
tumor progression
|
[12,16,71,72]
|
| |
|
cancer cells
|
tumor suppression
|
[29,40,73]
|
| |
|
immune cells
|
immune activation
|
[29,40,74]
|
|
sensory nerves
|
substance P
|
cancer cells
|
tumor progression
|
[34,75,76,77,78,79]
|
| |
|
endothelial cells
|
suppression of angiogenesis
|
[77,80]
|
| |
CGRP
|
endothelial cells
|
angiogenesis
|
[81]
|
| |
CCL/CXCL chemokines
|
immune cells
|
immune suppression
|
[82]
|
|
sympathetic/sensory nerves
|
serine
|
cancer cells
|
tumor progression
|
[83]
|
4. The Effect of Neural Signaling on Non-Tumor Cells
As mentioned above, autonomic neural signals can not only affect tumor cells but also other types of cells, especially immune cells. The inflammatory status in the body is regulated via humoral and neuronal pathways [
105,
106,
107].
4.1. Immune Cells
Associations between neuronal and immune systems have been reported to influence tumor immunity [
109,
110]. Neurogenic signatures were shown to be associated with immunosuppressive phenotypes [
111].
The function of T cells, especially cytotoxic CD8
+ T cells, is critical for anti-tumor immunity [
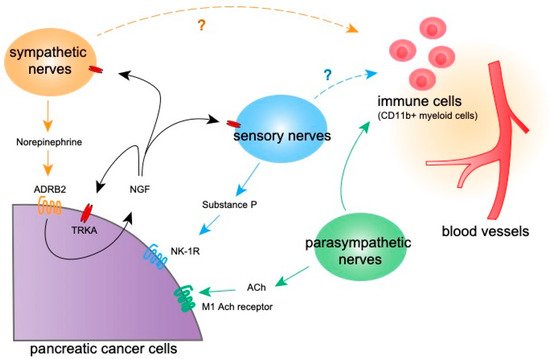
112]. Some studies have suggested that neural signaling plays a role in controlling anti-tumor T cell functions. The ablation of sympathetic nerves decreased programmed death-1 (PD-1) and FOXP3 expression on T cells in breast cancer [
29]. Accordingly, the parasympathetic stimulation of breast cancer cells decreased PD-L1 expression on tumor cells and PD-1 on T cells and increased CD8
+/regulatory T (Treg) cells [
29]. Another study demonstrated that the inhibition of β2-adrenergic receptor signaling on immune cells led to increased CD8
+ T cells and decreased PD-1 expression on T cells [
55]. In prostate cancer, PD-L1 expression on nerves in the tumor microenvironment was inversely correlated with the prevalence of CD8
+ T cells and patient prognosis [
113].
Macrophages infiltrating the tumor microenvironment are called tumor-associated macrophages (TAMs), which exert various effects to promote tumor initiation and progression [
114]. In breast cancer, β-adrenergic nerve stimulation induces infiltration and the differentiation of tumor-promoting macrophages in the tumor microenvironment, leading to tumor progression and angiogenesis [
56]. In contrast, cholinergic signaling suppresses the CD11b
+ myeloid cell population and TNFα expression in the PDAC microenvironment, indicating the tumor suppressive and anti-inflammatory effect of cholinergic signaling [
40]. Macrophages in the PDAC microenvironment are recruited by C-C chemokine receptor type 2 (CCR2) and colony-stimulating factors and secrete GDNFs to promote cancer migration and nerve invasion [
115].
Myeloid-derived suppressor cells (MDSCs) are activated neutrophils and monocytes which have immune suppressive functions to promote tumor progression [
116]. In melanoma, inhibition of β3-adrenergic receptor signaling attenuated regulatory T cells and MDSC increased the number and cytotoxicity of natural killer (NK) cells and increased the ratio of M1/M2 macrophages and N1 granulocytes [
57]. Sensory neurons have been reported to secrete several CCL and CXCL chemokines in the melanoma microenvironment, attracting MDSCs to promote immune-tolerant conditions [
82]. In colon cancer, cholinergic stimulation prevents colon cancer progression by inducing anti-inflammatory peptide trefoil factor 2 secretion from memory T cells to suppress MDSC expansion [
74].
NK cells also play an important role in innate tumor immunity [
117]. NK cells and nerves interact in the context of the degeneration of damaged sensory neurons through the NK cell receptor NKG2D and retinoic acid early-inducible 1 (RAE1) gene [
118]. Due to β2 adrenergic receptor signaling, NK cells and other leukocytes are mobilized into circulation [
119].
Eosinophils are granulocytes involved in innate immunity and have been shown to interact with neurons [
120]. Nerves recruit eosinophils through the stimulation of neuropeptides, cytokines, and chemokines; eosinophils release cationic proteins, neurotrophins/neuropeptides, and ROS to induce nerve growth and neuropeptide synthesis. In the tumor microenvironment, the role of eosinophils seems to be context-dependent [
121]. In some cancers, including melanoma, eosinophils exhibit anti-tumorigenic roles in mouse models, suggesting a novel therapeutic strategy.
4.2. Tumor Endothelial Cells (TECs)
Angiogenesis during tumor development has also been reported to be promoted by neural inputs. Vascular organization during development has been shown to be affected by sensory neurons [
122], as well as signaling via neuropeptide Y [
123]. In the tumor microenvironment, sympathetic nerve signaling induces angiogenesis, and TECs, in turn, promote tumorigenesis by secreting cytokines and growth factors [
58]. Systemic sympathetic nerve stimulation by the chronic restrain model revealed increased vascular endothelial growth factor (VEGF) expression and angiogenesis via β2-adrenergic receptor signaling in ovarian tumor cells [
14]. Catecholamines signaling through β-adrenergic receptors also induced expression of VEGF and IL-6 in breast cancer cells [
59]. In prostate cancer, β2-adrenergic receptor signaling on endothelial cells promoted tumor angiogenesis and tumor progression [
13], suggesting a mechanism involving immune regulation by sympathetic nerves through endothelial cells. In addition, catecholamine treatment induced the alternatively activated M2 polarization of macrophages to secrete VEGF and promote tumor angiogenesis in a lung cancer mouse model [
124]. On the other hand, dopamine, a neurotransmitter of sympathetic nerves, downregulates VEGF receptor 2 signaling in endothelial cells [
62] and inhibits colon cancer angiogenesis and growth [
63]. Similarly, gastric cancer and ovarian cancer mouse models have shown decreased tumor angiogenesis and tumor growth after dopamine treatment [
64,
65]. Collectively, in the tumor microenvironment, sympathetic innervation promotes angiogenesis supporting tumor progression.
4.3. Cancer-Associated Fibroblasts (CAFs)
CAFs are key components in the tumor microenvironment and have been extensively investigated and shown to have various functions, including modifying matrix deposition, reciprocal signaling, and interacting with cancer cells and immune cells to promote cancer progression [
125]. CAFs have also been shown to secrete several axon-guidance molecules. Exosomes derived from head and neck cancer cells induced NGF expression in fibroblasts [
126]. Pancreatic stellate cells also produce neurotrophic factors NGFs and artemin in response to transforming growth factor β (TGF-β) to induce neurite outgrowth [
127,
128,
129]. In pancreatic cancer, CAFs expressing NetrinG1, an axon-guidance molecule, have been shown to metabolically support tumor growth by affecting glutamate/glutamine metabolism and inhibiting NK-cell-mediated tumor killing via the Akt and p38 pathways [
130]. CAFs in the pancreatic cancer microenvironment have been reported to secrete an axon guidance molecule, SLIT2, to induce neural outgrowth [
131]. These studies suggest that CAFs are an important mediator of tumor innervation and neural remodeling in the tumor microenvironment.
4.4. Cancer-Associated Adipocytes(CAAs)
In adipocytes, β-adrenergic signaling, especially β3, is involved in the lipolytic mobilization of fatty acids [
132,
133]. In the cancer microenvironment, CAAs have been reported to promote tumor growth, angiogenesis, and migration through the secretion of hormones, cytokines, adipokines, and growth factors [
134].
5. Origins of Nerves in the Tumor Microenvironment
The mechanism of how neural cells expand in the tumor microenvironment is not clearly understood. One possibility is that pre-existing nerves directly innervate from the surrounding tissue. Co-culturing neural ganglia and cancer cells promote neurite outgrowth [
38,
39]. Such innervation might be induced by neurotrophins including nerve growth factors. Another possibility is the trans-differentiation of cells in the tumor microenvironment. Amit and colleagues reported that loss of
TP53 in oral cancer induced the trans-differentiation of tumor-associated sensory neurons into adrenergic neurons [
34]. Another study suggested the possibility of the trans-differentiation of cancer cells into neural cells in the tumor microenvironment of prostate cancer [
135]. Lastly, neural progenitor cells might be recruited to the tumor microenvironment from distant organs. Mauffrey and colleagues reported that doublecortin (DCX)-positive neural progenitors from the central nervous system infiltrated prostate primary tumors and metastases [
136].
6. The Molecular Mechanisms Involved in Nerve Expansion in the Tumor Microenvironment
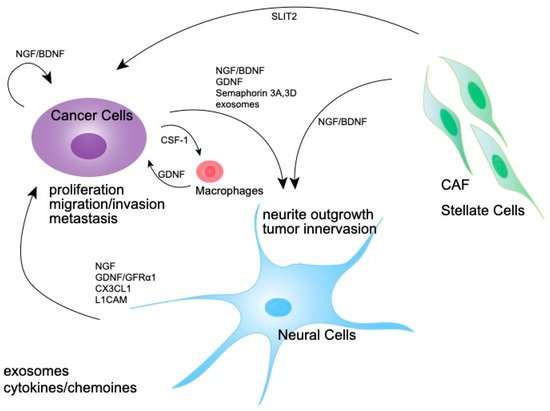
Various molecules, including neurotrophins, axon guidance molecules, and cytokines, are reportedly involved in the development and function of nerves in the tumor microenvironment (Figure 3).
Figure 3. Molecular mechanism of the expansion of tumor-associated nerves. A schematic diagram showing the interaction of nerves, pancreatic cancer cells, and CAFs via neurotrophins and other molecules which induce nerve growth.
7. Clinical Applications of Nerve-Targeting Therapy
Revealing the molecular mechanisms underlying nerves in the tumor microenvironment leads to novel therapeutic targeting, although only a limited number of molecular-targeting drugs have been approved in the field of tumor-associated nerves. Inhibitors of TRK receptors (pan-TRK inhibitors; entrectinib and larotrectinib) have been approved for solid tumors with TRK fusion [
179,
180,
181]. The effect of a multi-kinase inhibitor sitravatinib, which also inhibits Trk activity, on advanced solid tumors is currently being investigated (NCT02219711) [
182]. Although these drugs target TRK receptor signaling in cancer cells, they may exert inhibitory effects on innervation and tumor–nerve interactions in the tumor microenvironment, which should be determined in future studies.
Clinical trials to examine the effects of muscarinic agonists on PDAC (NCT03572283) and β-blockers in both non-metastatic and metastatic prostate and pancreatic cancer patients (NCT02944201, NCT03152786, NCT03838029, and NCT04245644) are ongoing. In addition, NK-1R antagonists have been suggested to exert anti-cancer effects both in a pre-clinical and clinical setting [
183]. Although these studies mainly target neural signaling in cancer cells, autonomic nerve signaling may also affect other targets including immune systems. In addition, CCR2 inhibitor treatment has been reported to enhance anti-tumor immunity in PDAC [
184], and has shown tolerability in PDAC patients [
185], possibly also having inhibitory effects on neural invasion or tumor innervation.
The most commonly observed serious treatment-related events were cognitive disorders in entrectinib [
180], suggesting a possible adverse effect on neural cells outside tumors by nerve-targeting drugs; moreover, muscarinic agonists or β-blockers may affect the cardiovascular function and bowel movements via autonomic nerve signaling. As such, for novel therapeutic agents targeting nerves, attention should be focused on avoiding adverse effects on normal neural activity.
Future studies should test multiple pathways and interactions in the tumor microenvironment discussed above. Surgical or pharmacological denervations of sympathetic/parasympathetic nerves or sensory neurons, and targeting immune–nerve or CAF–nerve interactions, may warrant future clinical studies. Overcoming suppressed anti-tumor immunity by modulating neural signaling may pave the way for novel immunotherapies in PDAC or other immunologically “cold” tumors. Metabolites secreted from nerves may also be an important therapeutic target, as the PDAC tumor microenvironment places a high demand on nutrients and might be dependent on the continuous supply of metabolites from stroma, including nerves.
Due to the complexity of the heterogeneity in the tumor microenvironment, determining an appropriate target is a critical process for developing novel therapeutic agents. Considering the higher incidence of perineural invasion and its impact on patient prognosis [
47,
48], it is important to elucidate the PDAC-specific mechanism by which PDAC becomes a more neurotropic tumor. One possibility may be the contribution of desmoplastic stroma, which characterizes PDAC. CAFs in PDAC have been shown to be a highly heterogeneous population [
186].