Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Medicine, Research & Experimental
Breast cancer treatment using plant-virus-based nanoparticles (PVNPs) has achieved considerable success in preclinical studies. PVNP-based breast cancer therapies include non-targeted and targeted nanoplatforms for delivery of anticancer therapeutic chemo and immune agents and cancer vaccines for activation of local and systemic antitumor immunity. Interestingly, PVNP platforms combined with other tumor immunotherapeutic options and other modalities of oncotherapy can improve tumor efficacy treatment.
- plant viruses
- in situ vaccination
- immunomodulatory agent
1. Introduction
Breast cancer (BC) is the most common form of cancer in women that is associated with abnormal tissue growth within the breasts and has the potential to metastasize to other areas of the body such as bone marrow and lungs [1]. BC could be classified as hormone-positive (estrogen receptor, ER+, and progesterone receptor, PR+), human epidermal receptor-2-positive (HER2+), and triple-negative BC (TNBC) (ER−, PR−, HER2−) [2,3]. Various modalities such as surgery (mastectomy and lumpectomy), chemotherapy, radiotherapy, hormonal therapy, and immunotherapy have been used for the treatment of BC [2]. A significant shortcoming associated with these therapies is the lack of complete efficiency due to the heterogeneity of tumor cell biology, the complexities of the tumor microenvironment (TME), and several host factors [2,4]. Thus, novel therapeutic strategies for the effective treatment of BC are urgently required.
A new promising strategy for the effective treatment of BC is the coupling of therapy modalities with nanotechnology [5]. Nanotechnology can provide effective therapies through the design of multifunctional nanoparticles (NPs) with variations in size, shape, composition, or surface chemistry and their potential for targeting tumor cells in a specific manner [4]. One type of nanotechnology discussed in BC therapy includes NPs which can be used as the nanocarriers for the delivery of a variety of antitumor payloads and bioactive compounds to enhance their solubility, circulatory half-life, and biodistribution while reducing their unwanted side effects [6]. Furthermore, NPs could be functionalized with targeting ligands that specifically bind to receptors on the surfaces of tumor cells and precisely target BC cells [7]. In this regard, various NPs with synthetic and natural origins have been developed, among them natural biological building-block-based NPs such as protein cages and viruses are a promising class [8].
As a type of nanoparticle, plant-virus-based nanoparticles (PVNPs) can become the leading tumor-therapy approach. PVNPs are two subsets, virus-like particles (VLPs) and virion nanoparticles (VNPs). VNPs are self-assembled nucleoprotein structures based upon identical coat proteins (CPs) and nucleic acid and can form various morphologies, the most common being icosahedral, road-shape, and filamentous [9,10].
Plant-virus-derived VLPs are proteins’ cage-like sphere structures lacking the viral genome [9,11]. Platform technologies based on PVNPs and VLPs are compelling due to [9,12,13]:
-
Their inherent safety, their non-infectious nature in mammals, biocompatibility, and biodegradability;
-
Their well-defined structural features, such as unique shapes and sizes, can be monodispersed for loading targeted molecular antitumor therapies onto their internal cavity and their interior and exterior surfaces by the assembly, infusion, or internal surface modification;
-
Their ability to self-assemble;
-
Their ability to be localized to the target site by chemical and genetic programmability;
-
Their simple and inexpensive production;
-
Their inherent immunogenicity enables them to act as nano-adjuvants and nanovaccines in cancer immunotherapy
In addition, morphological uniformity, water-solubility, and administration doses of up to 100 mg per kilogram body weight without signs of toxicity are unique advantages of PVNPs over other synthetic NPs [8,14].
2. Multifunctional PVNPs in Cancer Therapy
The use of PVPNs in tumor therapy research has been developed. They are widely used as therapeutic agent carriers by encapsulating and protecting them from degradation. The surface of PVNPs can be modified with the relevant ligands for targeting cancer cells (Figure 1A) [13]. These PVNPs can display the tumor-specific antigens as a vaccine and with exogenous adjuvant delivery can improve the efficiency of antitumor responses [12,13,15]. Furthermore, some PVNPs can act as adjuvants and with their monotherapy can modulate immunosuppressive TMEs via ligands for pattern recognition receptors (PRRs) of immune cells and induce the production of cytokines and chemokines [12,16].
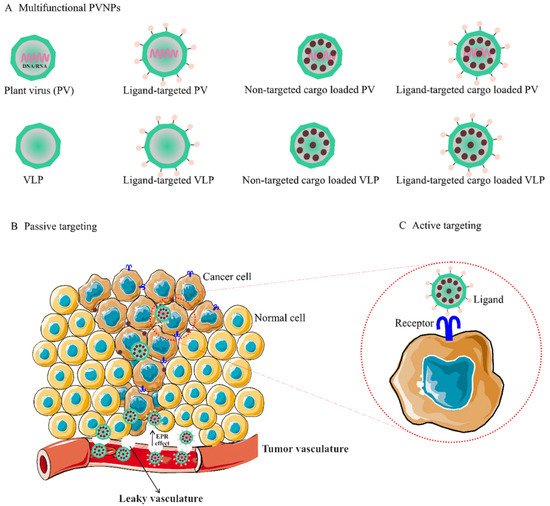
Figure 1. (A). Multifunctional PVNPs for breast cancer therapies. PVNPs/VLPs can functionalize by targeting ligands that specifically bind receptors on the surfaces of tumor cells. Non-targeted PVNP/VLP and targeted PVNP/VLP can load anticancer therapeutic agents. (B). Non-targeted PVNP/VLP can deliver the therapeutic agents into tumors via the enhanced permeation and retention (EPR) effect (passive targeting mechanism). (C). Targeted PVNP/VLP can deliver therapeutic agents via binding to tumor cells’ membrane-bound surface receptors (active targeting mechanism). Abbreviations: PVNPs, plant virus nanoparticles; VLP, virus-like particles.
Nanoengineering of PVNPs via noncovalent and covalent strategies has been developed specifically for loading and retaining therapeutic cargos and target molecules on PVNP surfaces. Noncovalent methods for packaging various cargoes rely on disassembly and reassembly to form uniform hollow containers (self-assembly method), electrostatic charges and hydrophobicity/hydrophilicity interactions (incubation method), and pores of “open” and “closed” states under certain environmental conditions (infusion method). Covalent-based cargo-loading methods take advantage of functional addressable groups (i.e., conjugation methods) and express simultaneously or separately by heterogeneous expression systems followed by self-assembly in vivo or in vitro (i.e., genetic methods) (reviewed in [8,9,12,13].
3. PVNP-Based BC Tumor Therapies
Similar to other solid tumors, the breast TME is composed of interactions between cellular and non-cellular compartments [17,18,19]. This TME acts as a barrier against current tumor treatment approaches and plays a critical role in BC development and progression as well as in determining the response to therapy [17]. PVNPs can be designed in a variety of ways to target these tumor barriers more importantly via the enhancing distribution of therapeutic agents into tumorous tissue and the fine-tuning of immunological responses [19]. The tumor targeting aspect of PVNPs relates to the inherent properties of PVNPs including [9,13]:
-
Nanoparticulate features such as composition, size, and surface properties;
-
Loading and targetability via nano-engineering;
-
Inherent immune stimulatory ability.
Moreover, the antitumor activity of PVNPs could be boosted by the inherent tumor properties including [20]:
-
The nature of tumor-associated vasculature in comparison to normal vasculature;
-
Overexpression of tumor-cell-based biomarkers in comparison to normal cells;
Overall, the current trend of PVNPs in BC treatment include: (1) the use of PVNPs as nanovehicles for non-targeted delivery (passive targeting) that rely on extravasation and increased concentration of PVNPs in tumors, (2) targeted delivery (active targeting), coupling PVNPs to targeting molecules overexpressed on tumor cells to efficiently infiltrate tumors and enter malignant cells, (3) tumor-targeted immunotherapies, and (4) combinational therapies.
3.1. PVNP-Based Non-Targeted Delivery for BC Tumor
Non-targeted delivery is reliant upon the composition, size (ranging from 50 to 200 nm), and surface properties of PVNPs, which all increase the permeability of the endothelial blood microvasculature of tumors, (i.e., enhanced permeation and retention (EPR) effect) [21]. For implementation, the particulate nature of PVNPs enables antitumor therapeutic payloads to be held within the interior cavity or on the exterior surface and become passively recruited to the tumors by the EPR effect (Figure 1C) [9,16]. In addition, PVNPs have blood and tissue compatibility, are stable under physiological conditions, and are less prone to interact with serum proteins compared to synthetic nanoparticles [22,23]. PVPNs are protein aqueous, imparting better cell penetration and endolysosomal escape than some synthetic NPs [22]. These properties of tumorous vascular and PVNPs improve passive targeted delivery and lead to the enhancement of the therapeutic index, the circulation time, the efficiency of delivery, and enhanced payload accumulation within solid tumors [24].
However, rapid blood clearance by reticuloendothelial system (RES) cells and protein corona effects can reduce the efficacy of non-targeted delivery of PVNPs before reaching the target site [25,26,27]. In most cases, the decoration of PVNPs with serum albumin or polyethylene glycol (PEG) results in a ‘camouflage’ effect, preventing their antibody recognition, thus enhancing their pharmacokinetics [28,29]. Furthermore, compared to their spherical counterparts, the shape of PVNPs is analogous to elongated architectures of nanomaterials, enhancing tumor homing and retention properties [30,31,32]. These properties can be due to increased margination toward the vessel wall, which will present ligands more effectively to target cells, and the flat vessel wall and are more likely to resist immune detection and macrophage uptake and contribute to longer circulation times, and beneficial flow properties. In addition, the short, cross-sectional dimension of nanorods determines membrane transfer efficiency [14,33]. Filamentous (e.g., potato virus X) or spherical (e.g., cowpea mosaic virus) shape mirrors the phenomenon that the human tumor xenografts exhibit higher uptake of PEGylated filamentous PVX compared to spherical CPMV [30].
Some antitumor therapeutic agents such as small molecule drugs, nucleic acid, and peptide/protein polymers loaded onto PVNPs have been used for delivery to breast tumors as summarized below.
3.1.1. Small Molecule Drug Delivery
The limitations of cancer treatment with small molecule drugs include poor bioavailability, drug resistance, recurrence, rapid drug clearance, non-targeted administration, high toxicities, and other side effects [34]. Therefore, various PVNPs have been developed and used for reducing these limitations. For example, loading mitoxantrone (MTO), a topoisomerase II inhibitor; phenanthriplatin, a cationic monofunctional DNA-binding platinum (II) complex; and gemcitabine, one of the nucleoside analog drugs, into tobacco mosaic virus (TMV) increased their accumulation within the tumor tissue and induced sufficient cytotoxicity toward MDA-MB-231, TNBC, and Michigan Cancer Foundation-7 (MCF7) cell lines [35,36]. In vivo MTO-TMV delivery in a mouse model of MDA-MB-231 xenografts reduced tumor growth, showing superior efficacy over free MTO [35]. Phenanthriplatin-loaded TMV in the MDA-MB-231-bearing mouse model has shown that the amount of drug within the tumor, when delivered by TMV, was increased ∼10-fold compared to the amount of drug administered systemically [37]. Furthermore, TMV-based spherical nanoparticles (SNPs), TMV, and Johnson grass chlorotic stripe mosaic virus (JgCSMV), loaded with doxorubicin showed a sustained drug release profile in BC cell lines (MDA-MB-231 and MCF-7) and breast tumor models [38,39]. Intraperitoneal (IP) injection of an MCF-7 tumor-bearing athymic mouse model with FA-JgCSMV-Dox tumor volumes were smaller when mice were treated with FA-JgCSMV-Dox compared to the controls [39]. Loading the prodrug 6-maleimidocaproyl-hydrazone doxorubicin (DOX-EMCH) with Physalis mottle virus (PhMV)-based VLPs and coating with PEG resulted in significantly great antitumor efficacy in vivo [40]. Overall, PVNPs present a programmable nano-scaffold-based platform for developing chemotherapeutics for BC models.
3.1.2. Nucleic Acid Delivery
Delivery of antitumor therapeutic nucleic acid polymers to BC can be limited due to extracellular and intracellular barriers such as poor cell uptake, instability in circulation in the presence of nucleases, and non-efficient delivery to their target site [23,41]. For example, ‘naked’ small interfering RNA (siRNA) is not stable in plasma, not targeted, and their negative charge impairs efficient cell uptake [42]. On the other hand, the use of nonviral nanocarriers composed of lipids or polymers often does not match the efficiency achieved using biological systems [23]. Therefore, the efficient delivery of this type of agent demands highly innovative systems [43]. The inherent nature of PVNPs to carry and deliver their genome into the host cells make them suitable as nanocarriers and an alternative or complementary approach for delivering therapeutic nucleic acids into tumor cells [43,44]. Importantly, they are preferentially taken up by innate immune cells such as macrophages (mainly M2 macrophages or tumor associates macrophages, TAM) and dendritic cells (DCs) in the TME [22] or can act as a toll-like receptor (TLR) agonist [45]. All these properties allow the PVNPs to be utilized to deliver therapeutic nucleic acids to innate immune cells in the treatment of cancer [8].
Recently, various PVNPs were used for encapsulation and delivery of nucleic acid polymers such as heterologous RNA, siRNAs, mRNA, and oligodeoxynucleotides (CpG-ODNs) into mammalian cells [22,23,42,46,47,48]. Akt 1 is a kinase involved in the processes of tumor cell proliferation and migration, and two examples, brome mosaic virus (BMV) and cowpea chlorotic mottle virus (CCMV) loaded with the antitumor siRNA Akt1 (siAkt1), have shown to be capable of being internalized by the tumor cells and thus, able to deliver the siAkt1 cargo into breast tumor cells [49]. CCMV formulated with siRNAs targeting FOXA1 (as a transcription factor of the Forkhead box (FOX) protein family) would allow gene silencing using the BC cell line MCF-7 [42]. In conclusion, besides enhancing the cellular uptake efficiency, the application of the PVNP-CP/VLP delivery system protected therapeutic nucleic acid from digestion while presenting better biocompatibility compared with the free formats [50].
3.1.3. Peptide/Protein Delivery
Amino acid polymer-based therapeutics are easy to synthesize, have high target specificity, and have low toxicity. When compared to conventional cancer treatments, amino acid polymer-based treatments are a promising novel approach to the treatment of cancer [51]. However, their application faced several drawbacks such as low stability, short half-life, and difficulties with encapsulation in vitro [8,51]. PVNPs can apply a framework for improving these problems via chemical or genetic fusion and self-assembly of the viral capsid protein around these therapeutic agents [51]. For example, Herceptin (trastuzumab), a humanized monoclonal antibody (mAb) that targets human epidermal growth factor receptor 2 (HER2)-positive suffers from a short half-life, structural heterogeneity, instability, and solubility limitations [44]. Herceptin or HER2 epitopes (CH401 epitope) conjugated with PVNPs such as PVX, Physalis mottle virus (PhMV), and CPMV have shown the promising targeting potentials of these platforms [52,53,54,55].
In addition, PVNPs can act as scaffolds for the delivery of proteinous drugs. For example, PVX, CPMV-Herceptin (trastuzumab) compared to free Herceptin significantly increases the rate of apoptosis in HER2 positive cell lines [52,56]. The conjugation of VEGFR-1 ligand and fluorescent PEGylated peptide on CPMV NPs can target VEGFR-1 on endothelial cell lines and VEGFR1-expressing tumor xenografts in vivo [57]. TRAIL, part of the tumor necrosis factor (TNF) superfamily is expressed as a homotrimeric type II transmembrane protein or under proteolytic cleavage converted into a soluble form [58]. TRAIL binds to the death receptors (DR4 and DR5) resulting in receptor trimerization and recruitment of FAS-associated protein with the death domain (FADD) to activate the extrinsic or caspase-dependent apoptosis in cancer cells (but not healthy cells) [59]. A short half-life, instability of the monomeric form of the TRAIL protein, and rapid renal clearance of the off-targeted TRAIL are the most significant obstacles to effectively triggering apoptosis in cancer cells [60]. Meanwhile, conjugating TRAIL protein to PVX could mimic the native TRAIL function, activate caspase-mediated apoptosis more efficiently compared to soluble TRAIL, and delay tumor growth in human TNBC xenografts [58].
3.2. PVNP-Based Targeted Delivery for BC Tumor
PVNPs have intrinsic ligands for binding to immune cells; however, they have no ligands on their surfaces for targeting and binding to cancer cells for payload delivery [8]. Thus, targeted ligands must be incorporated into the VLP or capsid scaffold of PVNPs for binding to specific receptors overexpressed on cancerous cells to maximize payload efficacy (Figure 2A). BC cells overexpress particular biomarkers such as the tyrosine kinase epidermal growth factor receptor (EGFR) (also known as ErbB-1 or HER-1), folate receptors (FR), and human HER2 [7]. Ligands of these receptors can conjugate to the surface of PVNP via nano-engineering to specifically target cancer cells. The mechanisms of ligand-based targeted PVNP for efficient treatment can include receptor-mediated endocytosis, receptor-mediated block, and receptor-mediated activation.
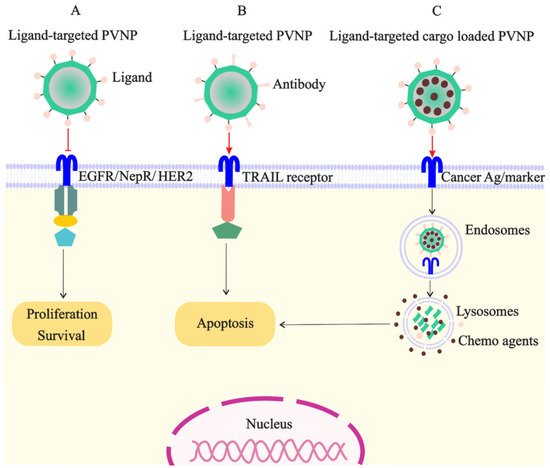
Figure 2. Targeted PVNP/VLP for treatment of breast cancer cells: (A). the ligands of receptors overexpressed on cancer cells (e.g., EGFR, NepR, and HER2) can link to PVNPs and inhibit cell survival and proliferation; (B). the ligand of TRAIL receptor overexpressed on cancer cells can link to PVNPs to induce apoptosis; (C). anticancer agents can load in targeted PVNPs for the internalization and delivery in the cancer cell, which subsequently induces cell death.
EGFR as the receptor tyrosine kinase (RTK)-based transmembrane receptor is overexpressed on the surface of BC cells (100 times more than normal cells) and also other solid tumors [7]. EGFR becomes activated with ligands of the EGF family and is internalized mostly via the clathrin-mediated pathway for triggering uncontrolled proliferation of cancer cells [61]. Therefore, EGFR is an ideal cancer biomarker for designing EGFR-based targeted therapies [62]. EGF-based mimetic ligands that do not activate EGFR-mediated signaling but are conjugated to PVNPs carrying toxic payloads is particularly promising [61]. Recently, GE11, a small peptide with 12 amino acids with imaging moieties (A647-PVX-GE11), was displayed on the surface of PVX and indicated suitable targeting and imaging EGFR+ MDA-MB-231 of ductal breast carcinoma (BT-474) cells [61,63].
This entry is adapted from the peer-reviewed paper 10.3390/vaccines10091431
This entry is offline, you can click here to edit this entry!
