Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Probiotic-based multi-component preparations refer to a mixture of bioactive agents, containing probiotics or postbiotics as main functional ingredients, and prebiotics, protectants, stabilizers, encapsulating agents, and other compounds as additional constituents.
- probiotics
- prebiotics
- synbiotics
- postbiotics
- human health
1. Introduction
During the 20th century, probiotics have been recognized and used as beneficial live microorganisms only for human and animal health [1]. A few years later, the concept of probiotics has been extended and applied to plant growth and protection, soil fertilizing [2], and depolluting [3]. Now, probiotics are considered to be a biotool par excellence that offers multiple potential solutions in improving human life for food and nutrition security [4], disease prevention [5][6][7][8], and environment protection [9][10]. Beside high functional interests, probiotics also benefit the material status of natural [11], safe or “generally recognized as safe” (GRAS) and “qualified presumption of safety” (QPS) [12], and renewable, i.e., cultivable and inexhaustible, biomass sources [13].
Probiotics are live microorganisms, mainly belonging to lactic acid bacteria (Lactobacilli), Bifidobacteria, soil-based bacteria (Bacillus sp.), and yeast (Saccharomyces sp.) groups [14] with different species and strains from food and non-food sources [15]. Their multiple functionalities in promoting human and ecosystem health result from their capacity to control pathogens, reduce toxin and polluting substances, and increase nutrient bioavailability through three main action mechanisms [16]. These include, among others: (1) surface and nutrient competition ability against pathogens through cell wall macromolecular structures (e.g., S-proteins and exopolysaccharides) and secreted amphiphilic compounds (e.g., biosurfactants); (2) antimicrobial production (e.g., bacteriocins, antiviral agents, enzymes, antioxidant compounds); and (3) immunomodulation activity to immune cells. In fact, probiotics act as immunomodulators by increasing the growth of healthy components and restoring the normal gut ecological niche [17]. Probiotics can stimulate phagocytic activity, balance pro- and anti-inflammatory cytokines, enhance the production of immunoglobulin (IgA) by plasma cells, and generate bioactive peptides.
From a technological viewpoint, probiotics are commonly produced by culture in fermenters, and used as functional ingredients in formulated food and non-food products, including fermented foods and beverages, diet supplements, drugs, and biological and cosmetic products [18]. Rarely used in pure forms, probiotics are mostly prepared and formulated with a wide range of other functional compounds for preserving, and even enhancing, cell performance, viability, and stability [19][20]. These components include thermo- and cryo-protectors [21], prebiotics [22], and encapsulating agents [23][24], or another probiotic species for preparing multi-strain products. When probiotics are combined with prebiotics, represented mainly by oligosaccharides, phenolic compounds, or polyunsaturated fatty acids, which serve as selective nutrients for probiotics [25], the multi-component preparations are called synbiotics.
When microbial cells are inactivated by thermal processing (e.g., pasteurization, tyndallization, autoclaving), and no longer contain viable probiotic cells, or the preparation consists of probiotic fragments or their metabolites, the term postbiotics is used [26].
The concept of “probiotic-based multi-components” is therefore more appropriate to design the preparations or formulations of probiotic multi-strains, synbiotics, or postbiotics, which are all beneficial for human, animal, and ecosystem health [27][28] when used under adequate conditions. In terms of analysis and characterization, the use only of gold-standard methods (e.g., genotype and phenotype profiling) is insufficient for identifying and fingerprinting all components, particularly the “additives” included in these probiotic-based multi-components. Other analytical and physical chemical tools are needed for identifying and quantifying, for instance, prebiotics and protectants in such formulations [29].
2. Probiotic-Based Multi-Components
2.1. Probiotics and Synbiotics
Probiotic-based multi-components are products that contain either one strain or a mixture of strains [30] and additional functional compounds. These are mainly thermo- or cryoprotectants [20][21], mainly carbohydrates (e.g., lactose, maltose, trehalose, maltodextrins), proteins (e.g., skim milk powder, whey protein), minerals (e.g., Ca++), and other compounds (e.g., glycerol), but also antioxidants such as ascorbic acid, tocopherol, and flavonoids [31]. Such additional compounds are essentially used to protect microbial cells against the changes in stress parameters such as temperature, pressure, oxygen exposure, and relative humidity, which cause losses in cell viability during the drying process of the probiotic culture and the storage of the resulting dry material [32][33]. Other functional compounds found in probiotic preparations are encapsulating agents when coating/encapsulation techniques are used for ensuring sufficient survival rates of microbial cells, until they reach the human or animal gut [34]. Among the current components used in such processes are hydrocolloid biopolymers, mainly constituted by proteins and polysaccharides, either for bulk or single-cell encapsulation systems [24]. According to the physical state of the product (dry or wet form), other extra additives and functional components used in probiotic formulations, for instance, anticaking agents, minerals, buffers, etc., can influence the performance of probiotic cells in terms of metabolic activity and survivability [35].
When prebiotics are combined with mono- or multi-strain probiotic preparations, the multi-component systems are called synbiotics [36]. Prebiotics are selective nutrients for beneficial microorganisms harbored by the host, mainly carbohydrate compounds such as inulin and fructo- and galacto-oligosaccharides (FOSs and GOSs), which are considered safe food ingredients in the European Union [25]. Moreover, as renewable and sustainable resources with a relatively low cost, these biocomponents appear to be eco-friendly and economically advantageous for use in agro-food sectors. Some foods and plant-based materials such as soybean extracts, kojiglycosylceramides, grape extracts, tea polyphenols, and seaweed extracts can also stimulate the proliferation of beneficial microorganisms in the intestine, and are considered as prebiotics [37]. Although all current prebiotics are carbohydrates, some polyphenols compounds have emerged and mediate beneficial physiological effects by modulating the gut microbiota [38].
Synbiotics as mixtures of probiotics and prebiotics can be designed in complement to independently target the host microorganisms, or in synergism, for which the prebiotic is selectively utilized by the co-administrated microorganisms to achieve one or more health benefits [36]. Consequently, such probiotic-based multi-components can be designed using a multitude of combinations with a wide range of properties and functions. The use of synbiotics with regards to the synergistic aspect confers them economic and environmental assets. Numerous benefits of synbiotics to human health have been shown [36] in comparison with those of animal [39] and plant cases in nutrition and health [40]. Synbiotic-based multi-components also appear to be relevant for promoting both food nutritional security and sustainable agriculture, due to their roles as biofertilizers and biopesticides [2]. Table 1 lists some common synbiotics with their components in probiotics and prebiotics, in addition to their commercial names.
Table 1. Some examples of synbiotics, their probiotic and prebiotic components, and trade names.
| Synbiotics | ||
|---|---|---|
| Probiotics | Prebiotics | Products |
| Lacticaseibacillus paracasei YIT 9029 (strain Shirota: LcS) Bifidobacterium breve YIT 12272 (BbrY) |
GOS | Super Synbiotics LBG-P (Yakult Honsha Co., Ltd., Tokyo, Japan) |
| Streptococcus thermophilus Lacticaseibacillus rhamnosus Lactobacillus acidophilus B. infantis B. lactis |
FOS + Ascorbic acid | Probiotical (Laboratoires Phacobel Belgium, Soheit-Tinlot, Belgium) |
| B. breve | Short chain scGOS Long chain lcFOS |
Danone Nutricia Research, Utrecht, The Netherlands |
| Bacillus coagulans MTCC 5856 | Cranberry fiber | Lactocran (Sabinsa Corporation, Piscataway, NJ, USA) |
| B. lactis, B. breve, B. infantis, B. longum | XOS | PrebioMed™ XOS (designs for health, Suffield, CT, USA) |
| Mix of Bifidobacteria and Lactobacilli | Whole fruit Indian Pomegranate (Punica granatum) (>40% Polyphenolic + Phenolic Bioactives) |
DS-01 (Seed Health, Los Angeles, CA, USA) |
| L. acidophilus Lacticaseibacillus casei, L. rhamnosus Lactiplantibacillus plantarum, S. thermophilus B. longum |
Oat Bran (10% β-Glucan fiber) Organic Red Beetroot Inulin from Organic Chicory Root |
Beta Glucan Synbiotic (BioImmersion Inc, Bellevue, WA, USA) |
| B. animalis, Enterococcus faecium, Limosilactobacillus reuteri Ligilactobacillus salivarius Pediococcus acidilactici |
Inulin | PoultryStar® (ME BIOMIN GmbH, Niederösterreich, Austria) |
| Enterococcus faecium | FOS | Biomin® IMBO (ME BIOMIN GmbH, Niederösterreich, Austria) |
| L. acidophilus L. casei L. salivarius L. plantarum, L. rhamnosus Levilactobacillus brevis B. bifidum B. lactis S. thermophilus |
Inulin | Synbiotic poultry (Vetafarm, Wagga Wagga, Australia) |
2.2. Postbiotics
The term “postbiotics” has been used for several years in different contexts, and the definition varies from one researchers to another, sometimes leading to confusion. Substances released by or produced through the metabolic activity of a microorganism that exerts a beneficial effect on the host, directly or indirectly, or substances of microorganism origin that confer beneficial effects to the host and differ from substances of a prebiotic nature, or non-viable probiotics, inanimate microorganisms and/or their components, paraprobiotics, and ghostbiotics, are among the terms currently used to name postbiotics [41][42]. A panel of scientist experts has declared that postbiotics are preparations of inanimate microorganisms and/or their components that confers a health benefit on the host [43]. The most recent concept of postbiotics uses the term “substances derived after the microorganisms are no longer alive, inanimate, dead or inactivated”, including intact cells or structural fragments of microbes such as the cell wall [26]. From a chemical viewpoint, postbiotics are heterogeneous multi-components of microbial metabolites from cell-free supernatants (e.g., supernatants of L. acidophilus, L. casei, L. rhamnosus GG, S. cerevisae, and S. boulardii cultures), or microbial fragments and lysates prepared by chemical and mechanical techniques such as sonication and heat treatments [42]. Metabolite-based postbiotics include exopolysaccharides (EPSs), e.g., β-glucan, antioxidant enzymes, short-chain fatty acids (SCFAs) such as acetic, propionic, and butyric acids, and vitamins; those from cell wall components are lipoteichoic acid, teichoic acids, peptidoglycan, cell surface proteins, and polysaccharides [44]. Some information on the potential applications of postbiotics for human health and their mechanisms of action related to antibacterial, antiviral, antioxidant, and anticancer activities have extensively been reviewed by different authors [45][46][47].
3. Applications and Action Mechanisms
3.1. Human Health
Humans are a reservoir of diverse group of microbes, which together constitute the human microbiome. This microbiome plays a key role in modulating the host internal environment, defending the body against infectious organisms and maintaining the health of humans [48]. The emergence of superbugs resistant to commonly used antibiotics suggests that the development of simple, low-cost, and intrinsic approaches to maintaining health are crucial. Probiotics have been shown to supplement the host microflora and protect against various pathogens by improving gut barrier function and activate specific genes in host cells, thereby stimulating the host’s immune response [49]. The gut microbiota in humans exert systemic effects on host health, metabolism, nutrition, and the immune system, which accounts for their designation as a “hidden metabolic organ” [50]. The evolution of the gut microflora from birth through adulthood is influenced by diet, genetic make-up, lifestyle and age of the host, and use of antibiotics [48]. Imbalances in the composition and function of intestinal microbes, referred to as gut dysbiosis, are associated with various human diseases [51]. Consequently, manipulation of intestinal microbiota, through diets that stimulate beneficial bacteria colonization of the GIT [52] and the administration of probiotics [53], holds promise for maintaining health and treatment of diseases. A shift from the healthy symbiosis between the microbiota and the host to persistent dysbiosis has also been identified as a factor in obesity [54]. Probiotics supplement host microflora and provide protection against various enteric pathogens, with demonstrated remarkable functional attributes for meeting most of the basic human nutritional and clinical supplementation requirements [48]. Although probiotics are essentially beneficial gut microorganisms, some species of probiotics are not part of the normal human gut flora, and the beneficial effects observed are not the same for different strains [55]. The majority of probiotics are species from three genera, viz., Lactobacillus, Bifidobacterium, and Saccharomyces [56][57][58]. The most-used vehicles for prebiotic administration have been pharmaceutical formulas and dairy products [59].
Probiotics have antipathogenic, antidiabetic, anti-obesity, anti-inflammatory, anticancer, anti-allergic, anti-anxiety, and angiogenic properties in humans [48]. These properties have been successfully harnessed to induce remission in ulcerative colitis [60] and reduce both weight and blood pressure [61]. Probiotics have also been shown to ameliorate infection and antibiotic-associated diarrhea, Clostridium difficile–associated diarrhea, and conditions such as allergic rhinitis and atopic dermatitis (eczema) [61]. Further research is required into the long-term utility and safety of probiotics in various disease conditions. Probiotics have been used to treat gastrointestinal (GI) and non-GI conditions that include traveler’s diarrhea, acute infectious diarrhea in infants and children, antibiotic-associated diarrhea, irritable bowel syndrome, and ulcer and atopic dermatitis [62][63], with effects also exercised on the brain and central nervous system [48][64] and cancer cells [65]. The advantages of probiotics are, however, more clearly demonstrated for GI-related diseases [62]. Probiotics significantly reduce the risk for diarrhea [66][67], with greater effectiveness obtained in children than adults [68]. Their effectiveness at reducing the frequency of antibiotic-associated diarrhea has also been demonstrated [67][69][70]. Probiotic strains L. fermentum NCMB 52221 and 8829 have shown considerable potency for suppressing colorectal cancer cells in vitro [65]. Probiotics and their fermented metabolites (postbiotics) have shown activities that counter oxidative stress, a factor in ageing, in middle-aged mice [59].
Probiotics have been trialed as a therapy for necrotizing enterocolitis (NEC). NEC is a serious inflammatory gastrointestinal disease that primarily affects premature infants and has a mortality rate as high as 50%. A Cochrane review with a meta-analysis of twenty-four eligible trials involving preterm infants <37 weeks gestational age or <2500 g birth weight showed that enteral probiotic supplementation significantly reduced the incidence of severe NEC (≥stage 2) and no systemic infection with the probiotic organism was reported in the trials [71]. Putative mechanisms for probiotic action in the gut include: (1) upregulation of cytoprotective genes; (2) competition with other microbes; (3) downregulation of pro-inflammatory gene expression; (4) production of butyrate and other short chain fatty acids that nourish colonocytes; (5) support of barrier maturation and function; and (6) regulation of cellular immunity and Th1:Th2 balance [61]. Outstanding issues to address include determining which probiotic to use, whether infants <1000 g benefit, and how to mitigate the risk of probiotic sepsis.
Research in animal models has shown that important components in mammalian milk, such as sialylated galacto-oligosaccharides (GOSs), reduce the occurrence of NEC in neonatal rats [72]. This could account for the 6–10-fold lower NEC risk in breast-fed infants compared to formula-fed infants. Indeed, GOSs appear to shape the components of the intestinal microbiome. Complex polysaccharides such as β-glucan (BGL) with anti-inflammatory properties have also shown promise in boosting growth performance and intestinal epithelium functions in weaned pigs, and hens [73][74]. More research is needed into the applicability of BGL in managing gastrointestinal inflammatory conditions such as NEC in humans.
Probiotics also play an important role in dentistry, since oral infections are considered prime among other infections affecting humans. Effects of probiotics on oral health are both direct and indirect. Some probiotics produce digestive enzymes for metabolizing proteins and carbohydrates. Several randomized clinical trials have shown the possible benefits of probiotic dairy products for oral health in children, adolescents, adults, and the elderly [59]. These studies indicate a role for probiotics in caries prophylaxis. The incorporation of probiotics into dairy products is due to their ability to neutralize acidic conditions that promote dental caries, the irreversible microbial disease of the calcified tissues of the teeth [75], and suppression of the caries pathogen. Given consumers’ concerns about allergens and lactose intolerance in respect of traditional dairy food matrices, there have been concerted efforts towards the development of cereals, soy, fruits, vegetables, and chocolate as innovative food matrices [76][77]. Although most probiotics are safe, they may sometimes come with side effects that include constipation, flatulence, hiccups, nausea, infection, and rashes [62]. In recent years, probiotic strains have been considered a powerful ally in fighting and preventing respiratory tract infections [63]. Reduction in upper and lower respiratory tract infections from the administration of probiotics bacteria has been reported [78]. The increasing evidence between gut and lung function, resulting from gut–lung cross-talk, suggests a possible role for probiotics in the management of COVID-19, caused by the severe acute respiratory syndrome corona virus 2 (SARS-CoV-2) that assumed a pandemic status in February 2020 [79].
In terms of action mechanisms, probiotics are involved in the maintenance of health through diverse and interconnected mechanisms. Probiotics produce vitamins, enhance nutrient absorption, and possess enzymatic activities, such as β-glucurodinase, β-galactosidase, and bile salt hydrolase, among other, that are essential for the host metabolism [61][80]. Probiotics modify microbiota populations through the production of short chain fatty acids (SCFAs), which alter luminal pH, and antimicrobial compounds, such as bacteriocin [81]. Probiotics stimulate the production of mucin glycoproteins and secretory immunoglobulin A (sIgA) by globet and B cells, respectively [81]. Mucin is necessary for probiotic adhesion to the intestinal mucosa, while impairing the adhesion of pathogen bacteria. sIgA serves as the first line of defense in protecting the gut from pathogens. Probiotics further modulate the immune system by interacting with toll-like receptors, thereby leading to the activation of the innate immune response; activating T-regulatory cells; and increasing the production of anti-inflammatory cytokines and reducing proinflammatory cytokines [61][82]. The production of SCFAs also plays an important role in the immune and inflammatory responses [83]. Moreover, SCFAs activate insulin sensitivity and fatty acid oxidation in muscle, decrease lipolysis and increase adipogenesis in adipose tissue, and enhance satiety through the stimulation of intestinal glucagon-like peptide 1 secretion [61][84]. The relevance of the gut microbiome on distal organs has led to defining the terms gut–brain, gut–lung, and gut–skin axes, among others. In nervous system disorders, the production of neuroactive compounds plays a significant role [85]. Finally, the interplay between the gut microbiome and other host microbiomes (lung, skin) is thought to contribute to the development of respiratory and skin diseases, in addition to the mitigation of symptoms [86][87][88][89]. Figure 1 illustrates the action mechanisms of probiotics for promoting human health.
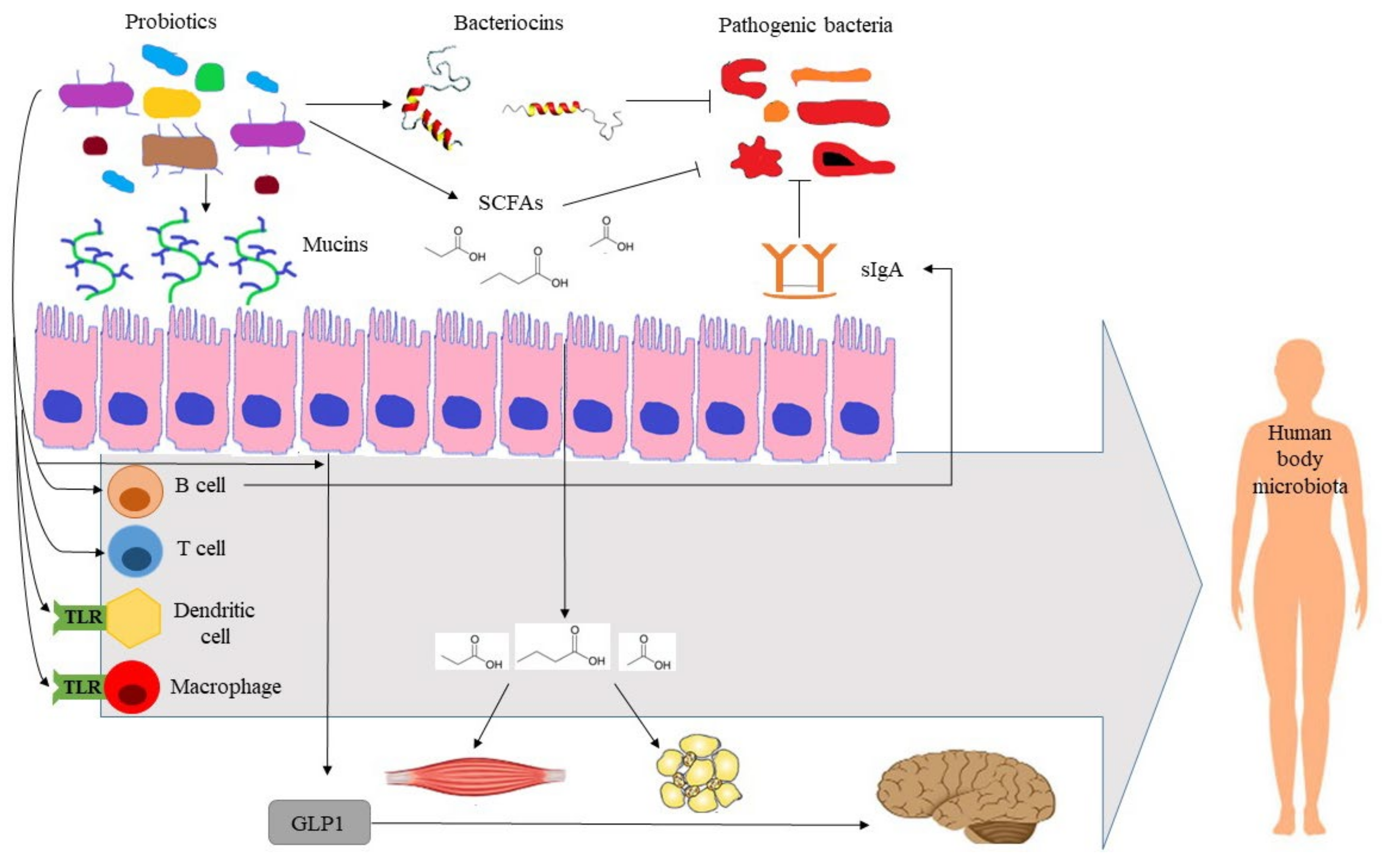
Figure 1. Illustration of action mechanisms of probiotics for promoting human health. GLP1: glucagon-like peptide 1, SCFAs: short-chain fatty acids, sIgA: soluble immunoglobulin A, TLR: toll-like receptor.
When probiotics are mixed with prebiotics, the resulting synbiotic preparation can develop either complementary or synergistic actions for human health [36]. Synbiotics help to manage several disease pathologies by targeting host gut microbiota, which play a crucial role in metabolism and protection against pathogens [90][91][92]. Synbiotics can act in balancing the gut microbiota by adjusting the Firmicutes-to-Bacteroidetes ratio, inhibiting harmful bacteria through direct antagonism (such as Klebsiella, Escherichia coli, and C. difficile) or excluding the latter by competitive adhesion, and accelerating the recovery to a healthy gut microbiome, e.g., by maintaining intestinal pH, producing important metabolites, and improving the gut mucosal barrier [93]. Health claims from clinical studies on synbiotics are linked to the gut health in order to treat inflammatory bowel syndrome (IBS) and inflammatory bowel disease (IBD), metabolic disease, and colorectal cancers. Other health claims relate to the treatment of systemic diseases such as allergies, hypocholesterolemia, osteoporosis, hepatic encelphalopathy; gut–brain axis diseases such as autism, depression, and anxiety [94]; and gut–lung axis respiratory diseases such as SARS-CoV-2 pathogenesis [95][96]. Some selected examples of synbiotic health benefits claimed from clinical studies are listed in Table 1. For further clinical trial results from studies conducted around the world, some databases are available online (https://www.clinicaltrials.gov/, accessed on 8 June 2021; https://www.clinicaltrialsregister.eu/, accessed on 1 January 2021).
Table 1. Example of human health benefits of synbiotics claimed from clinical studies.
| Health Outcomes | Probiotic Strains | Prebiotics | Reference | |
|---|---|---|---|---|
| Gut Intestinal tract | Treatment of overweight and metabolic syndrome | L. casei PXN 37, L. rhamnosus PXN 54, S. thermophilus PXN 66, B. breve PXN 25, L. acidophilus PXN 35, B. longum PXN 30, L. bulgaricus PXN 39 |
FOS | [97] |
| Treatment of IBS | Bacillus coagulans | FOS | [98] | |
| Acute diarrhea | L. acidophilus, L. rhamnosus, B. bifidum, B. longum, Enterococcus faecium |
FOS | [99] | |
| Colorectal cancer | B. lactis | Resistant starch | [100] | |
| Kidney | Treatment of chronic kidney disease |
L. casei, L. acidophilus, L. bulgaricus, L. rhamnosus, B. breve, B. longum, S. thermophilus |
FOS | [101] |
| Liver | Treatment of non-alcoholic fatty liver disease Prevention of infections after liver transplant |
B. longum L. acidophilus |
Inulin HP | [102] |
| Lung | Reduction of viral respiratory infections in asthmatic children | L. casei, L. rhamnosus, S. thermophilus, B. breve, L. acidophilus, B. infantis, L. bulgaricus |
FOS | [103] |
| Skin/derm | Treatment of atopic dermatitis | L. salivarius PM-A0006 | FOS | [104] |
| Brain | Improvement in mental health in hemodialysis patients | L. acidophilus strain T16, B. bifidum strain BIA-6, B. lactis strain BIA-7, B. longum strain BIA-8 |
Equal mix of FOS, GOS and inulin | [105] |
An alternative strategy to achieve the human health benefits of probiotics is the administration of bioactive compound-based preparations derived from probiotics, i.e., postbiotics [43]. They have several advantages over probiotics in terms of safety and production costs. Postbiotic health benefits rely on their antimicrobial, antioxidant, anticancer, and immunomodulatory potentials [44]. The postbiotic compounds with antimicrobial activities include bacteriocins and other peptides, SCFAs, organic acids, and hydrogen peroxide. The probiotic antioxidant enzymes catalase, superoxide dismutase, and glutathione peroxidase reduce the concentration of reactive oxygen species. Bacteriocins, and, in particular, enterocin, have cytostatic and apoptotic effects against cancer cells. The health-promoting effects of postbiotics include favoring mineral absorption, relieving constipation, preventing intestinal inflammation, controlling glycaemia, and reducing food allergies. Recent clinical trials have demonstrated the impact of postbiotics in a wide age range of individuals. In infants, the inclusion of postbiotics in an infant formula modifies the fecal microbiome and metabolome towards a profile closer to that observed in breast-fed infants [106]. In middle-aged individuals, the intake of urolithin A, a postbiotic metabolite of ellagitannins, improves muscle performance [107]. Figure 2 summarizes the potential applications of postbiotics in promoting human health.
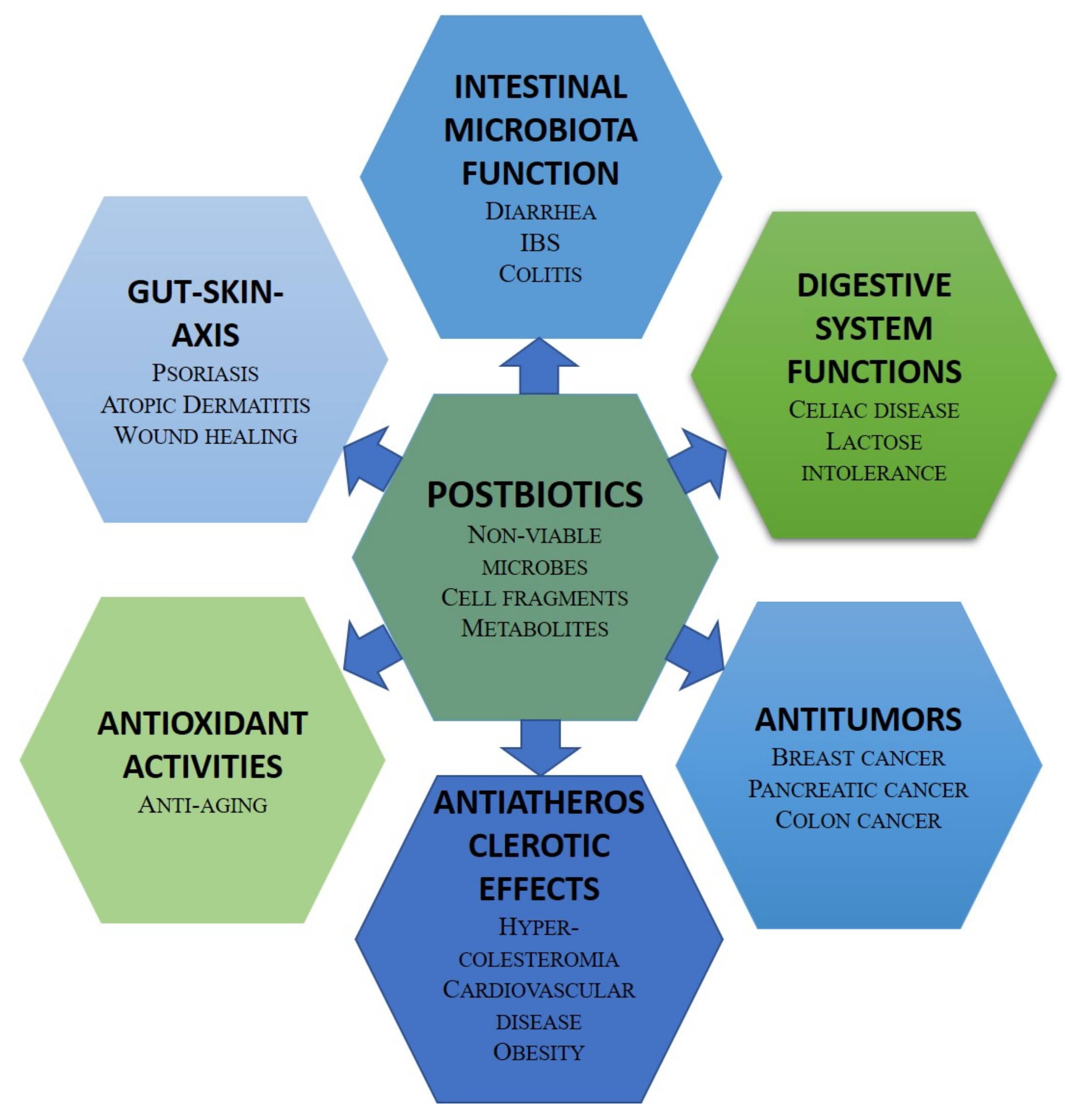
Figure 2. Potential applications of postbiotics in human health.
3.2. Animal Health
The main use of probiotics, prebiotics, postbiotics, and synbiotics in animal feeding is associated with their verified efficacy in modulation of the intestinal microbiota. Administration of probiotic strains, both individual and combined, may have a significant effect on absorption and utilization of feed, resulting in a daily increase in body weight and an increase in total body weight of various animals, including turkeys, chicken, piglets, sheep, goats, cattle, and horses. Probiotic microorganisms mostly intended for animals include Lactobacillus (e.g., L. brevis, L. casei, L. crispatus, L. farciminisa, L. fermentum), Bifidobacterium (e.g., B. animalis, B. longum, B. pseudolongum, B. thermophilum), other lactic acid bacteria (e.g., Enterococcus faecalis and faecium, Lactococcus lactis, Leuconostoc citreum, Pediococcus acidilactici) and some species of Bacillus, Saccharomyces, Kluyveromyces, and Aspergillus [108].
3.3. Plant and Soil Health
In the past decade, probiotics have been much applied to a wide range of industries such as aquaculture, food industries, human medicine, and agriculture. Some studies have been focused on successful practices, mechanisms of probiotics activities, and methods for optimizing the successful use of strains [109][110]. According to research results published in agriculture fields, the microbiome community known as probiotics can offer benefits to plant growth promotion, nutrient use efficiency (Figure 3), and pest and phytopathogen control [111][112]. Although many authors have demonstrated the interactions of probiotics with plants, a very little knowledge is available in the literature on the action mechanisms of prebiotics in the ecosystem. Results from research on forest ecosystems showed that fungal and bacterium communities can respond to environmental changes in accordance with host trees [113]. Vassilev et al. [114] demonstrated that Piriformospora indica, a beneficial microorganism for plants, can be used to produce a phosphate-enriched fermentation liquid through a repeated-batch fermentation process for improving soil fertility and plant productivity. In other work, it was proved that Bacillus amyloliquefaciens BChi1 and Paraburkholderia fungorum BRRh-4 can also increase growth and fruit yield of strawberries, and enhance their functional properties, such as the content of total antioxidants, carotenoids, flavonoids, phenolics, and anthocyanins [115]. In addition, other work demonstrated that microbial and biochemical indicators of soil health can be used to assess the ecological risk of soil. These results confirmed that soil respiration can be used for estimations of the soil ecological conditions and microbiological activity [116].
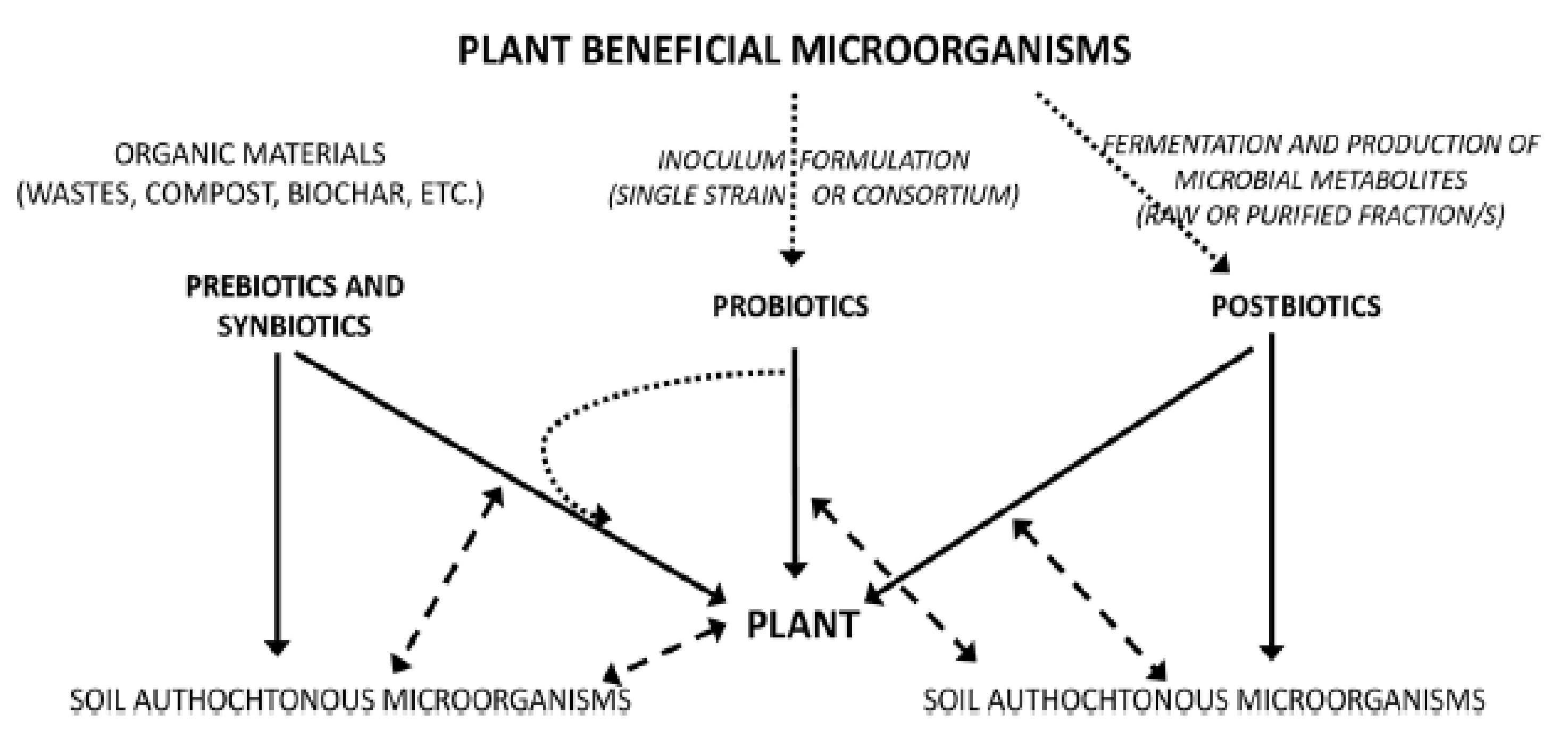
Figure 3. Diagram showing the three strategies for microbial soil–plant management based on prebiotics, probiotics, and postbiotics approaches. Legend: Full lines show the direct effect, dashed lines show the interactions, dotted lines show the formulation/production processes [117].
Basically, the ecosystem has been defined as a system of two components, constituting living organisms and inanimate or physical factors, respectively called biotic and abiotic components [118]. Biotic components comprise animals, microbial organisms, and plants, which are fed by nutrients, among which prebiotics and postbiotics constitute important elements. Prebiotics are molecules capable of stimulating both the intestinal microflora and other bacterial populations, including those growing in agricultural soils, by improving plant and soil health. Diverse sources of plant prebiotics such as fructo-oligosaccharides (FOSs), inulin, and galacto-oligosaccharides (GOS) are commonly cited, but xylans, pectins, and fructans are also among substrates used as carbohydrate-based prebiotics [119]. Information on postbiotics is very limited and associated research is quite recent. However, their role has been tested recently on animal, human, and plant health. It has been reported that postbiotics contribute to promoting plant growth by enhancing proliferation of shoots and rooting, and also having biocontrol effects on plants [117][120]. Limited studies on the effect of postbiotics on plants are available. Indeed, these derivate molecules from the plant probiotic microorganisms’ metabolism play mediating roles between probiotics and plants, acting as plant growth activators or in the defense of plants against certain stresses. Postbiotics interact via biochemical mechanisms with plant cellular membrane receptors through transduction of systemic signals, which leads to changes in plant gene expression at the plant level [121]. A large number of molecules obtained from probiotics activities act on plants, and significantly contribute to enhancing plant health performance, such as in terms of growth, yield, and resistance to stresses (biotic and abiotic).
3.4. Environmental Health
Probiotics play an important role as remediation agents, helping the host in responding to environmental changes. Certain genera also act as bioremediation or decomposing agents of hazardous substances [10], such as the case of a bacterial consortium (Xanthomonadaceae, Brachybacterium sp., Bhargavaea sp., Gordonia sp., Thalassospira sp., Pseudomonas sp., Dietzia sp., Mesorhizobium sp., Cytophaga sp., Martelella sp.), providing an innovative bioremediation approach. In this work, chitosan used as an encapsulated agent can stimulate the bacterial community of mangrove sediments [122]. Bioremediation in this case is based on the use of probiotics to degrade, reduce, or remove pollutants in the environment. The working mechanism of bioremediation involves several technical aspects such as biotransformation, biodegradation, mineralization, phytohydraulics, bioaccumulation, and biovolatilization, where the degrading microbes remove, transform, modify, and/or convert a complex compound of pollutants into simpler and less-toxic compounds. This bioremediation system has been successfully applied in cleaning contaminated sites [123], agricultural land [124], ground water [125], surface water [126], and sea water [127].
Conventional remediation strategies for most types of environmental contamination are not only expensive but also ineffective, especially in low contaminant concentrations [9]. Probiotics-assisted remediation has come forward as a cheap and easy alternative. Probiotics can act through four main action mechanisms divided into two categories, the binding and enzymatic degrading activities of toxins and pollutants, as summarized in Figure 4. LABs, yeasts, and soil probiotic bacteria are able to bind both organic toxins (e.g., mycotoxins and pesticides) and nonorganic pollutants (e.g., heavy metals) [3] through chelation, adsorption, and precipitation mechanisms. The nature and structure of the cell wall, surface macromolecules such as S-layer proteins and polysaccharides, and the environment conditions (e.g., pH and temperature), are among the factors that control the binding capacity of probiotics, which in turn depends on the surface hydrophobicity and electrical charge [3]. The binding mechanisms of toxins may also result from physical degradation of petroleum hydrocarbons [128]. Another mechanism is the production of enzyme-degrading toxins, such as organophosphorus-based pesticides [129], or proteolytic activity [130].
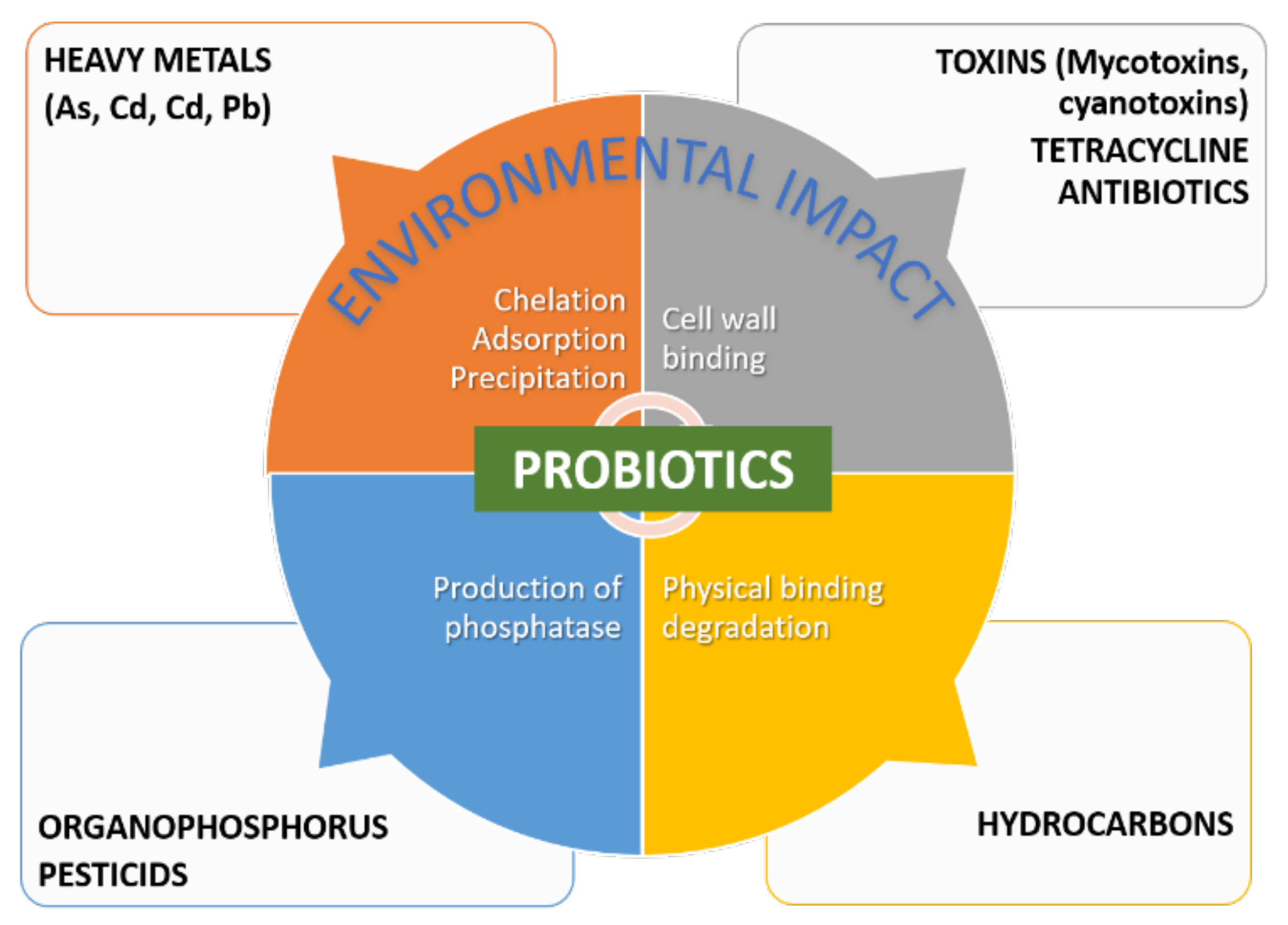
Figure 4. Main mechanisms of action of probiotics in remediation.
By combining probiotics with prebiotics, the resulting synbiotics are expected to develop a higher detoxifying capacity since prebiotics support the viability and functionalities of probiotics, which can improve the binding capacity of the mixture. The rare research work conducted on the synbiotic beneficial effects on bioremediation involved the combination of Lactobacilli and Bifidobacteria with inulin (prebiotic) for removing various substances, especially Pb [131].
Concerning postbiotics, it has been reported that both viable and nonviable LABs were able to bind toxic secondary metabolites such as fumonisin [132]. As the removal of mycotoxins involves an adhesion-type mechanism to cell wall components, rather than a covalent binding or binding by the metabolism, dead cells retain their binding ability [133]. Another case of postbiotic activity demonstrated in vivo was the removal of ochratoxin A from a liquid medium of foods using a mixture of sterilized yeast and a fermentation residue of beer (40:60). The binding action for toxin removal implied physical interactions with the cell wall since the changes in pH affected the degree of the activity [134].
This entry is adapted from the peer-reviewed paper 10.3390/microorganisms10091700
References
- Fuller, R. Probiotics in Man and Animals. J. Appl. Bacteriol. 1989, 66, 365–378.
- Song, D.; Ibrahim, S.; Hayek, S. Recent Application of Probiotics in Food and Agricultural Science. In Probiotics; Rigobelo, E., Ed.; InTech: London, UK, 2012; ISBN 978-953-51-0776-7.
- Zoghi, A.; Khosravi-Darani, K.; Sohrabvandi, S. Surface Binding of Toxins and Heavy Metals by Probiotics. Mini Rev. Med. Chem. 2014, 14, 84–98.
- Ebenso, B.; Otu, A.; Alessandro, G.; Philipe, C.; Victor, A.; Razafindralambo, H. Nature-Based One Health Approaches to Urban Agriculture Can Deliver Food and Nutrition Security. Front. Nutr. 2022, 9, 1–9.
- Lee, N.-K.; Paik, H.-D. Prophylactic Effects of Probiotics on Respiratory Viruses Including COVID-19: A Review. Food Sci. Biotechnol. 2021, 30, 773–781.
- Razafindralambo, H. Trends in Probiotic Applications; Studium Press LLC: Houston, TX, USA, 2018; ISBN 1-62699-110-3.
- Singh, K.; Rao, A. Probiotics: A Potential Immunomodulator in COVID-19 Infection Management. Nutr. Res. 2021, 87, 1–12.
- Soleymani, S.; Ebrahimi, F.; Rezaeizadeh, H.; Rahimi, R. Probiotics and Cancer. In Nutraceuticals and Cancer Signaling: Clinical Aspects and Mode of Action; Jafari, S.M., Nabavi, S.M., Silva, A.S., Eds.; Food Bioactive Ingredients; Springer International Publishing: Cham, Switzerland, 2021; pp. 467–527. ISBN 978-3-030-74035-1.
- Goyal, P.; Belapurkar, P.; Kar, A. A Review on in Vitro and in Vivo Bioremediation Potential of Environmental and Probiotic Species of Bacillus and Other Probiotic Microorganisms for Two Heavy Metals, Cadmium and Nickel. Biosci. Biotechnol. Res. Asia 2019, 16, 01–13.
- Helmy, Q.; Kardena, E.; Gustiani, S. Probiotics and Bioremediation. In Microorganisms; IntechOpen: London, UK, 2019.
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, Prebiotics and Synbiotics: Safe Options for next-Generation Therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521.
- Cavattoni, F. Probiotic Products: Technological and Regulatory Aspects. In Trends in Probiotic Applications; Hary Razafindralambo: Houxton, TX, USA, 2018; pp. 3–12.
- Cottet, C.; Ramírez Tapias, Y.A.; Delgado, J.F.; de la Osa, O.; Salvay, A.G.; Peltzer, M.A. Biobased Materials from Microbial Biomass and Its Derivatives. Materials 2020, 13, 1263.
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics Consensus Statement on the Scope and Appropriate Use of the Term Probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514.
- Rabetafika, H.-N.; Razafindralambo, A.; Razafindralambo, H. Probiotics from Food and Non-Food Sources. Trends Probiotic Appl. 2018, 53, 33–49.
- Oelschlaeger, T.A. Mechanisms of Probiotic Actions—A Review. Int. J. Med. Microbiol. 2010, 300, 57–62.
- Zambori, C.; Cumpănăşoiu, C.; Moţ, D.; Huţu, I.; Gurban, C.; Tîrziu, E. The Antimicrobial Role of Probiotics in the Oral Cavity in Humans and Dogs. Anim. Sci. Biotechnol. 2014, 47, 126–130.
- Muller, J.A.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Manufacture of Probiotic Bacteria. In Prebiotics and Probiotics Science and Technology; Springer: New York, NY, USA, 2009; Volume 1, pp. 725–759.
- Baral, K.C.; Bajracharya, R.; Lee, S.H.; Han, H.-K. Advancements in the Pharmaceutical Applications of Probiotics: Dosage Forms and Formulation Technology. Int. J. Nanomed. 2021, 16, 7535.
- Huang, S.; Vignolles, M.-L.; Chen, X.D.; Le Loir, Y.; Jan, G.; Schuck, P.; Jeantet, R. Spray Drying of Probiotics and Other Food-Grade Bacteria: A Review. Trends Food Sci. Technol. 2017, 63, 1–17.
- Su, Y.; Zheng, X.; Zhao, Q.; Fu, N.; Xiong, H.; Wu, W.D.; Chen, X.D. Spray Drying of Lactobacillus Rhamnosus GG with Calcium-Containing Protectant for Enhanced Viability. Powder Technol. 2019, 358, 87–94.
- Charalampopoulos, D.; Rastall, R.A. Prebiotics and Probiotics Science and Technology; Springer: New York, NY, USA, 2009; Volume 1.
- Burgain, J.; Gaiani, C.; Linder, M.; Scher, J. Encapsulation of Probiotic Living Cells: From Laboratory Scale to Industrial Applications. J. Food Eng. 2011, 104, 467–483.
- Centurion, F.; Basit, A.W.; Liu, J.; Gaisford, S.; Rahim, M.A.; Kalantar-Zadeh, K. Nanoencapsulation for Probiotic Delivery. ACS Nano 2021, 15, 18653–18660.
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502.
- Vinderola, G.; Sanders, M.E.; Salminen, S. The Concept of Postbiotics. Foods 2022, 11, 1077.
- Elko, N.; Foster, D.; Kleinheinz, G.; Raubenheimer, B.; Brander, S.; Kinzelman, J.; Kritzer, J.P.; Munroe, D.; Storlazzi, C.; Sutula, M.; et al. Human and Ecosystem Health in Coastal Systems. Shore Beach 2022, 90, 28.
- Vignieri, S.; Fahrenkamp-Uppenbrink, J. Ecosystem Earth. Science 2017, 356, 258–259.
- Razafindralambo, H. Advances in Physical Chemistry Tools for Probiotic Characterization. In Trends Probiotic Applications; Razafindralambo, H., Ed.; Studium Press LLC: Houston, TX, USA, 2018; pp. 50–81.
- Trush, E.A.; Poluektova, E.A.; Beniashvilli, A.G.; Shifrin, O.S.; Poluektov, Y.M.; Ivashkin, V.T. The Evolution of Human Probiotics: Challenges and Prospects. Probiotics Antimicrob. Proteins 2020, 12, 1291–1299.
- Niki, E. Free Radicals in the 1900’s: From in Vitro to in Vivo. Free Radic. Res. 2000, 33, 693–704.
- Barbosa, J.; Borges, S.; Teixeira, P. Influence of Sub-Lethal Stresses on the Survival of Lactic Acid Bacteria after Spray-Drying in Orange Juice. Food Microbiol. 2015, 52, 77–83.
- Iaconelli, C.; Lemetais, G.; Kechaou, N.; Chain, F.; Bermúdez-Humarán, L.G.; Langella, P.; Gervais, P.; Beney, L. Drying Process Strongly Affects Probiotics Viability and Functionalities. J. Biotechnol. 2015, 214, 17–26.
- Rajam, R.; Subramanian, P. Encapsulation of Probiotics: Past, Present and Future. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 46.
- Grumet, L.; Tromp, Y.; Stiegelbauer, V. The Development of High-Quality Multispecies Probiotic Formulations: From Bench to Market. Nutrients 2020, 12, 2453.
- Swanson, K.S.; Gibson, G.R.; Hutkins, R.; Reimer, R.A.; Reid, G.; Verbeke, K.; Scott, K.P.; Holscher, H.D.; Azad, M.B.; Delzenne, N.M. The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Synbiotics. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 687–701.
- Cheng, C.; Yu, X.; Geng, F.; Wang, L.; Yang, J.; Huang, F.; Deng, Q. Review on the Regulation of Plant Polyphenols on the Stability of Polyunsaturated-Fatty-Acid-Enriched Emulsions: Partitioning Kinetic and Interfacial Engineering. J. Agric. Food Chem. 2022, 70, 3569–3584.
- Bindels, L.B.; Delzenne, N.M.; Cani, P.D.; Walter, J. Towards a More Comprehensive Concept for Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 303–310.
- Malik, J.K.; Prakash, A.; Srivastava, A.K.; Gupta, R.C. Synbiotics in Animal Health and Production. In Nutraceuticals in Veterinary Medicine; Springer: Cham, Switzerland, 2019; pp. 287–301.
- Tabacchioni, S.; Passato, S.; Ambrosino, P.; Huang, L.; Caldara, M.; Cantale, C.; Hett, J.; Del Fiore, A.; Fiore, A.; Schlüter, A. Identification of Beneficial Microbial Consortia and Bioactive Compounds with Potential as Plant Biostimulants for a Sustainable Agriculture. Microorganisms 2021, 9, 426.
- Tsilingiri, K.; Rescigno, M. Postbiotics: What Else? Benef. Microbes 2013, 4, 101–107.
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A Step beyond Pre-and Probiotics. Nutrients 2020, 12, 2189.
- Salminen, S.; Collado, M.C.; Endo, A.; Hill, C.; Lebeer, S.; Quigley, E.M.M.; Sanders, M.E.; Shamir, R.; Swann, J.R.; Szajewska, H.; et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 649–667.
- Rad, A.H.; Hosseini, S.; Pourjafar, H. Postbiotics as Dynamic Biological Molecules for Antimicrobial Activity: A Mini-Review. Biointerface Res. Appl. Chem. 2022, 12, 6543–6556.
- Abbasi, A.; Sheykhsaran, E.; Kafil, H.S. Postbiotics: Science, Technology and Applications; Bentham Science Publishers: Sharjah, United Arab Emirates, 2021; ISBN 1-68108-839-8.
- Aghebati-Maleki, L.; Hasannezhad, P.; Abbasi, A.; Khani, N. Antibacterial, Antiviral, Antioxidant, and Anticancer Activities of Postbiotics: A Review of Mechanisms and Therapeutic Perspectives. Biointerface Res. Appl. Chem. 2021, 12, 2629–2645.
- Wegh, C.A.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673.
- Kerry, R.G.; Patra, J.K.; Gouda, S.; Park, Y.; Shin, H.-S.; Das, G. Benefaction of Probiotics for Human Health: A Review. J. Food Drug Anal. 2018, 26, 927–939.
- Rao, S.C.; Athalye-Jape, G.K.; Deshpande, G.C.; Simmer, K.N.; Patole, S.K. Probiotic Supplementation and Late-Onset Sepsis in Preterm Infants: A Meta-Analysis. Pediatrics 2016, 137, e20153684.
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; Abd El-Wahab, A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395.
- Illiano, P.; Brambilla, R.; Parolini, C. The Mutual Interplay of Gut Microbiota, Diet and Human Disease. FEBS J. 2020, 287, 833–855.
- Bottari, B.; Quartieri, A.; Prandi, B.; Raimondi, S.; Leonardi, A.; Rossi, M.; Ulrici, A.; Gatti, M.; Sforza, S.; Nocetti, M. Characterization of the Peptide Fraction from Digested Parmigiano Reggiano Cheese and Its Effect on Growth of Lactobacilli and Bifidobacteria. Int. J. Food Microbiol. 2017, 255, 32–41.
- Yan, F.; Polk, D.B. Probiotics and Immune Health. Curr. Opin. Gastroenterol. 2011, 27, 496.
- Breton, J.; Galmiche, M.; Déchelotte, P. Dysbiotic Gut Bacteria in Obesity: An Overview of the Metabolic Mechanisms and Therapeutic Perspectives of next-Generation Probiotics. Microorganisms 2022, 10, 452.
- Senok, A.C.; Ismaeel, A.Y.; Botta, G.A. Probiotics: Facts and Myths. Clin. Microbiol. Infect. 2005, 11, 958–966.
- Mizock, B.A. Probiotics. Dis. -A-Mon. DM 2015, 61, 259–290.
- Pandey, K.R.; Naik, S.R.; Vakil, B.V. Probiotics, Prebiotics and Synbiotics-a Review. J. Food Sci. Technol. 2015, 52, 7577–7587.
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The Pros, Cons, and Many Unknowns of Probiotics. Nat. Med. 2019, 25, 716–729.
- Da Cruz, M.F.; Magno, M.B.; Jural, L.A.; Pimentel, T.C.; Masterson, D.; Esmerino, E.A.; Ramos, G.L.P.A.; Gomila, J.V.; Silva, M.C.; Cruz, A.G. Probiotics and Dairy Products in Dentistry: A Bibliometric and Critical Review of Randomized Clinical Trials. Food Res. Int. 2022, 157, 111228.
- Ganji-Arjenaki, M.; Rafieian-Kopaei, M. Probiotics Are a Good Choice in Remission of Inflammatory Bowel Diseases: A Meta Analysis and Systematic Review. J. Cell. Physiol. 2018, 233, 2091–2103.
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of Action of Probiotics. Adv. Nutr. 2019, 10, S49–S66.
- Islam, S.U. Clinical Uses of Probiotics. Medicine 2016, 95, e2658.
- Liu, Y.; Tran, D.Q.; Rhoads, J.M. Probiotics in Disease Prevention and Treatment. J. Clin. Pharmacol. 2018, 58, S164–S179.
- Tillisch, K. The Effects of Gut Microbiota on CNS Function in Humans. Gut Microbes 2014, 5, 404–410.
- Kahouli, I.; Malhotra, M.; Alaoui-Jamali, M.; Prakash, S. In-Vitro Characterization of the Anti-Cancer Activity of the Probiotic Bacterium Lactobacillus Fermentum NCIMB 5221 and Potential against Colorectal Cancer. J. Cancer Sci. 2015, 7, 224–235.
- Hilton, E.; Kolakowski, P.; Singer, C.; Smith, M. Efficacy of Lactobacillus GG as a Diarrheal Preventive in Travelers. J. Travel Med. 1997, 4, 41–43.
- McFarland, L.V. Meta-Analysis of Probiotics for the Prevention of Traveler’s Diarrhea. Travel Med. Infect. Dis. 2007, 5, 97–105.
- Sazawal, S.; Hiremath, G.; Dhingra, U.; Malik, P.; Deb, S.; Black, R.E. Efficacy of Probiotics in Prevention of Acute Diarrhoea: A Meta-Analysis of Masked, Randomised, Placebo-Controlled Trials. Lancet Infect. Dis. 2006, 6, 374–382.
- Cremonini, F.; Di Caro, S.; Nista, E.C.; Bartolozzi, F.; Capelli, G.; Gasbarrini, G.; Gasbarrini, A. Meta-Analysis: The Effect of Probiotic Administration on Antibiotic-Associated Diarrhoea. Aliment. Pharmacol. Ther. 2002, 16, 1461–1467.
- Naidoo, K.; Gordon, M.; Fagbemi, A.O.; Thomas, A.G.; Akobeng, A.K. Probiotics for Maintenance of Remission in Ulcerative Colitis. Cochrane Database Syst. Rev. 2011, CD007443.
- AlFaleh, K.; Anabrees, J. Probiotics for Prevention of Necrotizing Enterocolitis in Preterm Infants. Evid.-Based Child Health A Cochrane Rev. J. 2014, 9, 584–671.
- Autran, C.A.; Schoterman, M.H.; Jantscher-Krenn, E.; Kamerling, J.P.; Bode, L. Sialylated Galacto-Oligosaccharides and 2′-Fucosyllactose Reduce Necrotising Enterocolitis in Neonatal Rats. Br. J. Nutr. 2016, 116, 294–299.
- Vetvicka, V.; Oliveira, C. β(1-3)(1-6)-D-Glucans Modulate Immune Status in Pigs: Potential Importance for Efficiency of Commercial Farming. Ann. Transl. Med. 2014, 2, 16.
- Jacob, J.P.; Pescatore, A.J. Barley β-Glucan in Poultry Diets. Ann. Transl. Med. 2014, 2, 20.
- Alok, A.; Singh, I.D.; Singh, S.; Kishore, M.; Jha, P.C.; Iqubal, M.A. Probiotics: A New Era of Biotherapy. Adv. Biomed. Res. 2017, 6, 31.
- Fiocco, D.; Longo, A.; Arena, M.P.; Russo, P.; Spano, G.; Capozzi, V. How Probiotics Face Food Stress: They Get by with a Little Help. Crit. Rev. Food Sci. Nutr. 2020, 60, 1552–1580.
- Pimentel, T.C.; da Costa, W.K.A.; Barão, C.E.; Rosset, M.; Magnani, M. Vegan Probiotic Products: A Modern Tendency or the Newest Challenge in Functional Foods. Food Res. Int. 2021, 140, 110033.
- Campbell, K. How Some Probiotic Scientists Are Working to Address COVID-19. 2020. Available online: https://isappscience.org/how-some-probiotic-and-prebiotic-scientists-are-working-to-address-covid-19/ (accessed on 29 June 2022).
- Bottari, B.; Castellone, V.; Neviani, E. Probiotics and COVID-19. Int. J. Food Sci. Nutr. 2021, 72, 293–299.
- Markowiak, P.; Śliżewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021.
- Van Zyl, W.F.; Deane, S.M.; Dicks, L.M.T. Molecular Insights into Probiotic Mechanisms of Action Employed against Intestinal Pathogenic Bacteria. Gut Microbes 2020, 12, 1831339.
- Burgueño, J.F.; Abreu, M.T. Epithelial Toll-like Receptors and Their Role in Gut Homeostasis and Disease. Nat Rev Gastroenterol Hepatol 2020, 17, 263–278.
- Mirzaei, R.; Dehkhodaie, E.; Bouzari, B.; Rahimi, M.; Gholestani, A.; Hosseini-Fard, S.R.; Keyvani, H.; Teimoori, A.; Karampoor, S. Dual Role of Microbiota-Derived Short-Chain Fatty Acids on Host and Pathogen. Biomed. Pharmacother. 2022, 145, 112352.
- Blaak, E.E.; Canfora, E.E.; Theis, S.; Frost, G.; Groen, A.K.; Mithieux, G.; Nauta, A.; Scott, K.; Stahl, B.; van Harsselaar, J.; et al. Short Chain Fatty Acids in Human Gut and Metabolic Health. Benef. Microbes 2020, 11, 411–455.
- Lai, Y.; Dhingra, R.; Zhang, Z.; Ball, L.M.; Zylka, M.J.; Lu, K. Toward Elucidating the Human Gut Microbiota–Brain Axis: Molecules, Biochemistry, and Implications for Health and Diseases. Biochemistry 2021.
- Fabbrizzi, A.; Amedei, A.; Lavorini, F.; Renda, T.; Fontana, G. The Lung Microbiome: Clinical and Therapeutic Implications. Intern. Emerg. Med. 2019, 14, 1241–1250.
- Gutiérrez-Castrellón, P.; Gandara-Martí, T.; Abreu, Y.; Abreu, A.T.; Nieto-Rufino, C.D.; López-Orduña, E.; Jiménez-Escobar, I.; Jiménez-Gutiérrez, C.; López-Velazquez, G.; Espadaler-Mazo, J. Probiotic Improves Symptomatic and Viral Clearance in Covid19 Outpatients: A Randomized, Quadruple-Blinded, Placebo-Controlled Trial. Gut Microbes 2022, 14, 2018899.
- Mercader-Barceló, J.; Truyols-Vives, J.; Río, C.; López-Safont, N.; Sala-Llinàs, E.; Chaplin, A. Insights into the Role of Bioactive Food Ingredients and the Microbiome in Idiopathic Pulmonary Fibrosis. Int. J. Mol. Sci. 2020, 21, 6051.
- Sinha, S.; Lin, G.; Ferenczi, K. The Skin Microbiome and the Gut-Skin Axis. Clin. Dermatol. 2021, 39, 829–839.
- Khan, I.; Ullah, N.; Zha, L.; Bai, Y.; Khan, A.; Zhao, T.; Che, T.; Zhang, C. Alteration of Gut Microbiota in Inflammatory Bowel Disease (IBD): Cause or Consequence? IBD Treatment Targeting the Gut Microbiome. Pathogens 2019, 8, 126.
- Li, H.-Y.; Zhou, D.-D.; Gan, R.-Y.; Huang, S.-Y.; Zhao, C.-N.; Shang, A.; Xu, X.-Y.; Li, H.-B. Effects and Mechanisms of Probiotics, Prebiotics, Synbiotics, and Postbiotics on Metabolic Diseases Targeting Gut Microbiota: A Narrative Review. Nutrients 2021, 13, 3211.
- Sergeev, I.N.; Aljutaily, T.; Walton, G.; Huarte, E. Effects of Synbiotic Supplement on Human Gut Microbiota, Body Composition and Weight Loss in Obesity. Nutrients 2020, 12, 222.
- Kvakova, M.; Bertkova, I.; Stofilova, J.; Savidge, T.C. Co-Encapsulated Synbiotics and Immobilized Probiotics in Human Health and Gut Microbiota Modulation. Foods 2021, 10, 1297.
- Krumbeck, J.A.; Walter, J.; Hutkins, R.W. Synbiotics for Improved Human Health: Recent Developments, Challenges, and Opportunities. Annu. Rev. Food Sci. Technol. 2018, 9, 451–479.
- Ahmadi Badi, S.; Tarashi, S.; Fateh, A.; Rohani, P.; Masotti, A.; Siadat, S.D. From the Role of Microbiota in Gut-Lung Axis to SARS-CoV-2 Pathogenesis. Mediat. Inflamm. 2021, 2021, 6611222.
- Antunes, A.E.; Vinderola, G.; Xavier-Santos, D.; Sivieri, K. Potential Contribution of Beneficial Microbes to Face the COVID-19 Pandemic. Food Res. Int. 2020, 136, 109577.
- Eslamparast, T.; Zamani, F.; Hekmatdoost, A.; Sharafkhah, M.; Eghtesad, S.; Malekzadeh, R.; Poustchi, H. Effects of Synbiotic Supplementation on Insulin Resistance in Subjects with the Metabolic Syndrome: A Randomised, Double-Blind, Placebo-Controlled Pilot Study. Br. J. Nutr. 2014, 112, 438–445.
- Rogha, M.; Esfahani, M.Z.; Zargarzadeh, A.H. The Efficacy of a Synbiotic Containing Bacillus Coagulans in Treatment of Irritable Bowel Syndrome: A Randomized Placebo-Controlled Trial. Gastroenterol. Hepatol. Bed Bench 2014, 7, 156.
- Dinleyici, E.C.; Dalgic, N.; Guven, S.; Ozen, M.; Kara, A.; Arica, V.; Metin-Timur, O.; Sancar, M.; Kurugol, Z.; Tanir, G. The Effect of a Multispecies Synbiotic Mixture on the Duration of Diarrhea and Length of Hospital Stay in Children with Acute Diarrhea in Turkey: Single Blinded Randomized Study. Eur. J. Pediatrics 2013, 172, 459–464.
- Worthley, D.L.; Le Leu, R.K.; Whitehall, V.L.; Conlon, M.; Christophersen, C.; Belobrajdic, D.; Mallitt, K.-A.; Hu, Y.; Irahara, N.; Ogino, S. A Human, Double-Blind, Placebo-Controlled, Crossover Trial of Prebiotic, Probiotic, and Synbiotic Supplementation: Effects on Luminal, Inflammatory, Epigenetic, and Epithelial Biomarkers of Colorectal Cancer. Am. J. Clin. Nutr. 2009, 90, 578–586.
- Dehghani, H.; Heidari, F.; Mozaffari-Khosravi, H.; Nouri-Majelan, N.; Dehghani, A. Synbiotic Supplementations for Azotemia in Patients with Chronic Kidney Disease: A Randomized Controlled Trial. Iran. J. Kidney Dis. 2016, 10, 351–357.
- Javadi, L.; Khoshbaten, M.; Safaiyan, A.; Ghavami, M.; Abbasi, M.M.; Gargari, B.P. Pro-and Prebiotic Effects on Oxidative Stress and Inflammatory Markers in Non-Alcoholic Fatty Liver Disease. Asia Pac. J. Clin. Nutr. 2018, 27, 1031–1039.
- Ahanchian, H.; Jafari, S.A.; Ansari, E.; Ganji, T.; Kiani, M.A.; Khalesi, M.; Momen, T.; Kianifar, H. A Multi-Strain Synbiotic May Reduce Viral Respiratory Infections in Asthmatic Children: A Randomized Controlled Trial. Electron. Physician 2016, 8, 2833.
- Wu, K.-G.; Li, T.-H.; Peng, H.-J. Lactobacillus Salivarius plus Fructo-Oligosaccharide Is Superior to Fructo-Oligosaccharide Alone for Treating Children with Moderate to Severe Atopic Dermatitis: A Double-Blind, Randomized, Clinical Trial of Efficacy and Safety. Br. J. Dermatol. 2012, 166, 129–136.
- Haghighat, N.; Mohammadshahi, M.; Shayanpour, S.; Haghighizadeh, M.H.; Rahmdel, S.; Rajaei, M. The Effect of Synbiotic and Probiotic Supplementation on Mental Health Parameters in Patients Undergoing Hemodialysis: A Double-Blind, Randomized, Placebo-Controlled Trial. Indian J. Nephrol. 2021, 31, 149.
- Rodriguez-Herrera, A.; Tims, S.; Polman, J.; Porcel Rubio, R.; Muñoz Hoyos, A.; Agosti, M.; Lista, G.; Corvaglia, L.T.; Knol, J.; Roeselers, G. Early-Life Fecal Microbiome and Metabolome Dynamics in Response to an Intervention with Infant Formula Containing Specific Prebiotics and Postbiotics. Am. J. Physiol.-Gastrointest. Liver Physiol. 2022, 322, G571–G582.
- Singh, A.; D’Amico, D.; Andreux, P.A.; Fouassier, A.M.; Blanco-Bose, W.; Evans, M.; Aebischer, P.; Auwerx, J.; Rinsch, C. Urolithin A Improves Muscle Strength, Exercise Performance, and Biomarkers of Mitochondrial Health in a Randomized Trial in Middle-Aged Adults. Cell Rep. Med. 2022, 3, 100633.
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Girones, R.; Herman, L.; Koutsoumanis, K.; Lindqvist, R.; Nørrung, B.; et al. Scientific Opinion on the Update of the List of QPS-recommended Biological Agents Intentionally Added to Food or Feed as Notified to EFSA. EFSA J. 2017, 15, e04664.
- Batista, K.S.; de Albuquerque, J.G.; de Vasconcelos, M.H.A.; Bezerra, M.L.R.; da Silva Barbalho, M.B.; Oliveira, R.P.; de Souza Aquino, J. Probiotics and Prebiotics: Potential Prevention and Therapeutic Target for Nutritional Management of COVID-19? Nutr. Res. Rev. 2021, 1–42.
- McKenzie, V.J.; Kueneman, J.G.; Harris, R.N. Probiotics as a Tool for Disease Mitigation in Wildlife: Insights from Food Production and Medicine. Ann. N. Y. Acad. Sci. 2018, 1429, 18–30.
- Ray, P.; Lakshmanan, V.; Labbé, J.L.; Craven, K.D. Microbe to Microbiome: A Paradigm Shift in the Application of Microorganisms for Sustainable Agriculture. Front. Microbiol. 2020, 11, 622926.
- Haque, M.M.; Mosharaf, M.K.; Khatun, M.; Haque, M.A.; Biswas, M.S.; Islam, M.S.; Islam, M.M.; Shozib, H.B.; Miah, M.M.U.; Molla, A.H. Biofilm Producing Rhizobacteria with Multiple Plant Growth-Promoting Traits Promote Growth of Tomato under Water-Deficit Stress. Front. Microbiol. 2020, 11, 542053.
- Park, K.H.; Oh, S.-Y.; Yoo, S.; Fong, J.J.; Kim, C.S.; Jo, J.W.; Lim, Y.W. Influence of Season and Soil Properties on Fungal Communities of Neighboring Climax Forests (Carpinus cordata and Fraxinus rhynchophylla). Front. Microbiol. 2020, 11, 2643.
- Vassilev, N.; Eichler-Löbermann, B.; Flor-Peregrin, E.; Martos, V.; Reyes, A.; Vassileva, M. Production of a Potential Liquid Plant Bio-Stimulant by Immobilized Piriformospora Indica in Repeated-Batch Fermentation Process. AMB Express 2017, 7, 1–7.
- Rahman, M.; Sabir, A.A.; Mukta, J.A.; Khan, M.; Alam, M.; Mohi-Ud-Din, M.; Miah, M.; Rahman, M.; Islam, M.T. Plant Probiotic Bacteria Bacillus and Paraburkholderia Improve Growth, Yield and Content of Antioxidants in Strawberry Fruit. Sci. Rep. 2018, 8, 1–11.
- Niemeyer, J.C.; Lolata, G.B.; de Carvalho, G.M.; Da Silva, E.M.; Sousa, J.P.; Nogueira, M.A. Microbial Indicators of Soil Health as Tools for Ecological Risk Assessment of a Metal Contaminated Site in Brazil. Appl. Soil Ecol. 2012, 59, 96–105.
- Vassilev, N.; Vassileva, M.; Martos, V.; Garcia del Moral, L.F.; Kowalska, J.; Tylkowski, B.; Malusá, E. Formulation of Microbial Inoculants by Encapsulation in Natural Polysaccharides: Focus on Beneficial Properties of Carrier Additives and Derivatives. Front. Plant Sci. 2020, 11, 270.
- González-Salazar, C.; Stephens, C.R.; Marquet, P.A. Comparing the Relative Contributions of Biotic and Abiotic Factors as Mediators of Species’ Distributions. Ecol. Model. 2013, 248, 57–70.
- Dwivedi, S.; Sahrawat, K.; Puppala, N.; Ortiz, R. Plant Prebiotics and Human Health: Biotechnology to Breed Prebiotic-Rich Nutritious Food Crops. Electron. J. Biotechnol. 2014, 17, 238–245.
- Moradipour, M.; Saberi-Riseh, R.; Mohammadinejad, R.; Hosseini, A. Nano-Encapsulation of Plant Growth-Promoting Rhizobacteria and Their Metabolites Using Alginate-Silica Nanoparticles and Carbon Nanotube Improves Ucb1 Pistachio Micropropagation. J. Microbiol. Biotechnol. 2019, 29, 1096–1103.
- Harman, G.; Khadka, R.; Doni, F.; Uphoff, N. Benefits to Plant Health and Productivity from Enhancing Plant Microbial Symbionts. Front. Plant Sci. 2021, 11, 610065.
- Angelim, A.L.; Costa, S.P.; Farias, B.C.S.; Aquino, L.F.; Melo, V.M.M. An Innovative Bioremediation Strategy Using a Bacterial Consortium Entrapped in Chitosan Beads. J. Environ. Manag. 2013, 127, 10–17.
- Yadav, K.K.; Gupta, N.; Kumar, V.; Singh, J.K. Bioremediation of Heavy Metals from Contaminated Sites Using Potential Species: A Review. Indian J. Environ. Prot. 2017, 37, 65.
- Baez-Rogelio, A.; Morales-García, Y.E.; Quintero-Hernández, V.; Muñoz-Rojas, J. Next Generation of Microbial Inoculants for Agriculture and Bioremediation. Microb. Biotechnol. 2017, 10, 19–21.
- Farhadian, M.; Vachelard, C.; Duchez, D.; Larroche, C. In Situ Bioremediation of Monoaromatic Pollutants in Groundwater: A Review. Bioresour. Technol. 2008, 99, 5296–5308.
- Cao, W.; Zhang, H.; Wang, Y.; Pan, J. Bioremediation of Polluted Surface Water by Using Biofilms on Filamentous Bamboo. Ecol. Eng. 2012, 42, 146–149.
- Voskoboinikov, G.M.; Matishov, G.G.; Metelkova, L.O.; Zhakovskaya, Z.A.; Lopushanskaya, E.M. Participation of the Green Algae Ulvaria Obscura in Bioremediation of Sea Water from Oil Products. In Proceedings of the Doklady Biological Sciences; Pleiades Publishing: New York, NY, USA, 2018; Volume 481, pp. 139–141.
- Fragoso ados Santos, H.; Duarte, G.A.S.; Rachid, C.T.d.C.; Chaloub, R.M.; Calderon, E.N.; Marangoni, L.F.d.B.; Bianchini, A.; Nudi, A.H.; Do Carmo, F.L.; van Elsas, J.D. Impact of Oil Spills on Coral Reefs Can Be Reduced by Bioremediation Using Probiotic Microbiota. Sci. Rep. 2015, 5, 1–11.
- Sarlak, Z.; Khosravi-Darani, K.; Rouhi, M.; Garavand, F.; Mohammadi, R.; Sobhiyeh, M.R. Bioremediation of Organophosphorus Pesticides in Contaminated Foodstuffs Using Probiotics. Food Control 2021, 126, 108006.
- Nybom, S.M.K.; Dziga, D.; Heikkilä, J.E.; Kull, T.P.J.; Salminen, S.J.; Meriluoto, J.A.O. Characterization of Microcystin-LR Removal Process in the Presence of Probiotic Bacteria. Toxicon 2012, 59, 171–181.
- Mirza Alizadeh, A.; Hosseini, H.; Mohseni, M.; Eskandari, S.; Sohrabvandi, S.; Hosseini, M.-J.; Tajabadi-Ebrahimi, M.; Mohammadi-Kamrood, M.; Nahavandi, S. Analytic and Chemometric Assessments of the Native Probiotic Bacteria and Inulin Effects on Bioremediation of Lead Salts. J. Sci. Food Agric. 2021, 101, 5142–5153.
- Niderkorn, V.; Boudra, H.; Morgavi, D.P. Binding of Fusarium Mycotoxins by Fermentative Bacteria In Vitro. J. Appl. Microbiol. 2006, 101, 849–856.
- Sampaio Baptista, A.; Horii, J.; Antonia Calori-Domingues, M.; Micotti da Glória, E.; Mastrodi Salgado, J.; Roberto Vizioli, M. The Capacity of Manno-Oligosaccharides, Thermolysed Yeast and Active Yeast to Attenuate Aflatoxicosis. World J. Microbiol. Biotechnol. 2004, 20, 475–481.
- Shetty, P.H.; Hald, B.; Jespersen, L. Surface Binding of Aflatoxin B1 by Saccharomyces Cerevisiae Strains with Potential Decontaminating Abilities in Indigenous Fermented Foods. Int. J. Food Microbiol. 2007, 113, 41–46.
This entry is offline, you can click here to edit this entry!
