Curcumin has multiple properties that are used to cure different diseases such as cancer, infections, inflammatory, arthritic disease, etc. Despite having many effects, the inherent physicochemical properties—such as poor water solubility, chemical instability, low bioavailability, photodegradation, fast metabolism, and short half-life—of curcumin’s derivatives have limited its medical importance. Mesoporous silica nanoparticles (MSNs) are promising drug delivery systems for more effective and safer treatment of several diseases, such as infections, cancers, and osteoporosis. Achieving a high drug loading in MSNs is critical to the success of this type of treatment. Their notable inherent properties—such as adjustable size and porosity, high pore volume, large surface area, functionality of versatile surfaces, as well as biocompatibility—have prompted extraordinary research on MSNs as multi-purpose delivery platforms.
1. Mesoporous Silica Nanoparticles for Co-Delivery of Curcumin with Antibiotics
First, the ability of mesoporous silica nanoparticles (MSNs) to inter the bacteria and biofilm has been illustrated, then the challenge is in fighting antibacterial resistance by designing nanosystems with improved antimicrobial effectiveness through combining different antimicrobial components in the same nanoplatforms. For this purpose, various studies have identified innovative strategies based on shared delivery of antibiotics or combining antibiotics and antimicrobial metal nanoparticles or ions
[1][2].
Concerning the first approach, the easiest way is to load different antibiotic molecules into the MSNs’ pores at the same time. The co-loading of agents with different—and sometimes conflicting—physicochemical properties in 26-side a single MSN requires development of compartmentalization approaches. One of the interesting methods for co-delivery of therapeutic agents of different chemicals is the use of MSNs with asymmetric structure and anisotropic geometry and two compartments that can load drugs of different natures in separate storage spaces. Song et al. decorated silver nanoparticles in mesoporous silica of SBA-15 covered by melanin-like polydopamine (PDA) as carriers. During this time, the constructed mesopores were charged with curcumin (CCM) by their noncovalent connections with coatings of PDA. The acquired CCM@SBA-15/PDA/Ag compounds were characterized physiochemically and demonstrated low hemolytic action and positive biocompatibility
[3]. Realizing the dual-stimuli-responsive (ROS and pH) from silver and/or curcumin nanoparticles from the composites of CCM@SBA-15/PDA/Ag was carried out to decrease the side effects of the drug leakage of non-controlled due to physiological circumstances. Moreover, in comparison to that of CCM@SBA-15/PDA and SBA-15/PDA/Ag, CCM@SBA-15/PDA/Ag combination displayed a long-lasting inhibitive effect on the growth of
S. aureus (24 h) and
E. coli (72 h), attributed to the increased impact of the bactericide of curcumin and silver nanoparticles
[3].
Asymmetric MSNs with dual-compartments and anisotropic geometry are very desirable for charging and releasing dual drugs into separated storage areas. In a study, an asymmetric lollipop-like mesoporous silica nanoparticle Fe
3O
4@SiO
2&EPMO (EPMO = ethane bridged periodic mesoporous organosilica) was extended effectively through an anisotropic epitaxial growth approach. The absorbance measurement at 600 nm evaluated the antibacterial effects of Fe
3O
4@SiO
2-GS&EPMO-Cur.
S. aureus and
E. coli were selected as bacterial models. At the same concentration, Fe
3O
4@SiO
2&EPMO-Cur and Fe
3O
4@SiO
2-GS& EPMO-Cur have a better antimicrobial effect than curcumin, because of the enhanced solubility of curcumin loaded with the asymmetric lollipop-like nanoparticles. The rising concentration of curcumin, Fe
3O
4@SiO
2&EPMO-Cur, and Fe
3O
4@SiO
2-GS&EPMO-Cur caused the viability of
E. coli and
S. aureus to decrease considerably. In addition, Fe
3O
4@SiO
2-GS&EPMO-Cur shows that it has much more capacity for inhibiting bacteria than Fe
3O
4@SiO
2&EPMO-Cur in the same concentration. This is due to the synergic effect of curcumin and gentamicin concurrently loaded into the asymmetric lollipop-like nanoparticles
[4].
2. Antibacterial Effect of Curcumin-Loaded Mesoporous Silica Nanoparticles (MSNs)
The antibacterial activity of curcumin on Gram-negative and Gram-positive species has been demonstrated. Cheng and coworkers designed novel core–shell magnetic MSNs. Curcumin and gentamicin were separately loaded into the hydrophobic and hydrophilic spaces to provide dual anticancer and antibacterial nanosystems
[4].
A trio-hybrid nanocomposite of MSNs including copper, loaded with curcumin and decorated with Ag nanoparticles, represented the photokilling of
E. coli [5]. The nanocomposite displayed a positive surface load, and Ag attendance raised the production of ROS. After irradiation, the trio-hybrids with curcumin at 1.5 µM diminished the viability of bacteria by 5 and 4 log in comparison with free curcumin and nanocomposite without curcumin, respectively. At 3 µM of curcumin, the bacterial cells were destroyed by the trio-hybrids. Nanocomposite-mediated aPDT bacterial cell damage is shown in SEM images
[5]. A nanocomposite film of chitosan and curcumin-loaded MSNs was prepared and indicated an antibacterial effect against
E. coli and
S. aureus, which was evaluated by the disk diffusion test
[6]. It has been seen that the inhibitory effect on
S. aureus was more than
E. coli; however, the antibacterial activity of chitosan with curcumin was more than the nanocomposite film
[6].
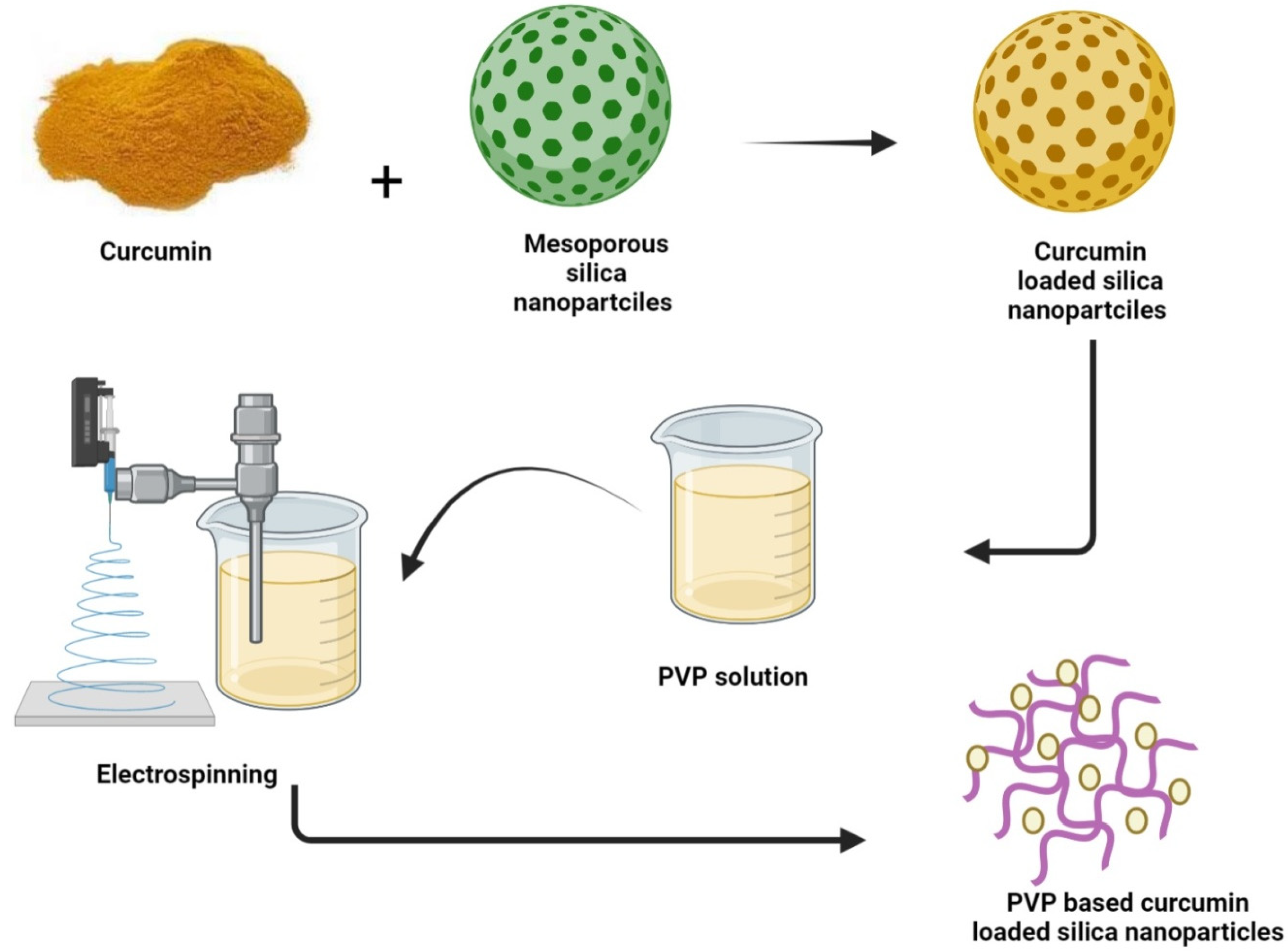
To combine the benefits of polyvinylpyrrolidone (PVP) with MSNs, Li et al. have designed a hybrid hemostatic organic–inorganic material using electrospinning by integrating the curcumin-loaded MSNs (CCM-MSNs) into PVP nanofiber mats (
Figure 1). Furthermore, increased antibacterial activities against methicillin-resistant
S. aureus (MRSA) were represented by hybrid nanofiber mats in vitro
[7].
Figure 1. Fabrication of curcumin-loaded PVP based silica mesoporous nanofibers mat.
Curcumin is loaded into already prepared mesoporous silica nanoparticles and the resultant complex is introduced into a PVP (polyvinylpyrrolidone) solution. Electrospinning of the admixture leads to the drug arrangement into suitable positions of the polymer results in the production of hybrid PVP-based curcumin-loaded mesoporous silica nanofibers mat.
Kuthati et al. prepared copper-loaded MSNs (Cu-MSNs) with immobilized silver nanoparticles (SNPs) for applying photodynamic inactivation (PDI) to drug-resistant E. coli. SNPs were decorated over the surfaces of Cu-MSNs by coordination of silver ions on diamine-functionalized Cu-MSNs and with formalin further reduced to silver nanoparticles. The ability of silver in sensitizing E. coli as a specific phototherapeutic agent in vitro was shown, so it can expand the phototherapeutic spectrum of curcumin. After external decoration with silver nanoparticles, and following loading of curcumin, the mesoporous structure of Cu-MSNs remains intact. Various physical characterization techniques have confirmed the synthesis and successful assembly of the functional nanomaterials. Under light irradiation, curcumin may produce large quantities of ROS, which may enhance the kinetics of silver ion release for antimicrobial activity. Cu-MSNs positive-laden modified surfaces support antimicrobial response by electrostatic attractions to negative-load bacterial cell membranes.
A synergic mechanism for transferring energy of the absorbed light from SNP to curcumin may activate the antibacterial effect of the prepared nanocomposites
[5]. Vallet-Regí and co-workers—who were working on bone infections—encountered a challenge in designing and developing advanced nano-antibiotics
[1].
First, bacteria that are present in infections of bone-implant (both in the planktonic and biofilm forms) should be locally destroyed in situ to prevent the need for surgical procedures for removing and replacing prostheses that were infected by bacteria. In contrast, in the infected area, antimicrobials—which may contain only antibiotics or the combination of metal ions and antibiotics—must be treated with small, high-specificity doses to enhance their efficiency and decrease dangerous side effects in healthy cells, tissues, and organs.
This aim can be achieved via nanocarriers capable of charging, protecting, and transporting antimicrobial agents to the target (free bacterial and biofilm form). Upon arrival, the nanocarriers gradually release antimicrobial agents in response to specific external or internal stimuli. Among nanosystems, MSNs came as the third item that meets all those demands. In this way, innovative and advanced technologies are used in the design and development of advanced targeted stimuli-responsive MSNs-based nanocarriers for antimicrobials administration.
3. Antiviral Effect
Several investigations have been performed to study the antiviral potential of the herbal compound due to the lack of prevention and treatment options for numerous viral infections
[8][9]. The curcumin showed antiviral effects against different viruses such as influenza viruses, hepatitis viruses, and chikungunya virus (CHIKV); or emerging arboviruses such as Zika virus (ZIKV)
[10]. Interestingly, curcumin decreases the spread of sexually transmitted diseases such as human papillomavirus (HPV), human immunodeficiency virus (HIV), and herpes simplex virus 2 (HSV-2)
[11][12][13]. Mesoporous material made from an iron–phenanthroline nanocomplex was utilized to encapsulate curcumin (NCIP) with antiretroviral effect against HIV-1 human microglial cells
[14]. Immunofluorescence staining displayed that NCIP decreased HIV-p24 expression by 41%, while free curcumin decreased by 24%, which showed suppression of HIV replication. NCIP showed an anti-inflammatory effect with a decrease in interleukin-8 (IL-8), nitric oxide, and TNF-α expression. Antioxidant activity was also observed, as NCIP decreased expression of heme oxygenase and increased expression of catalase
[14].
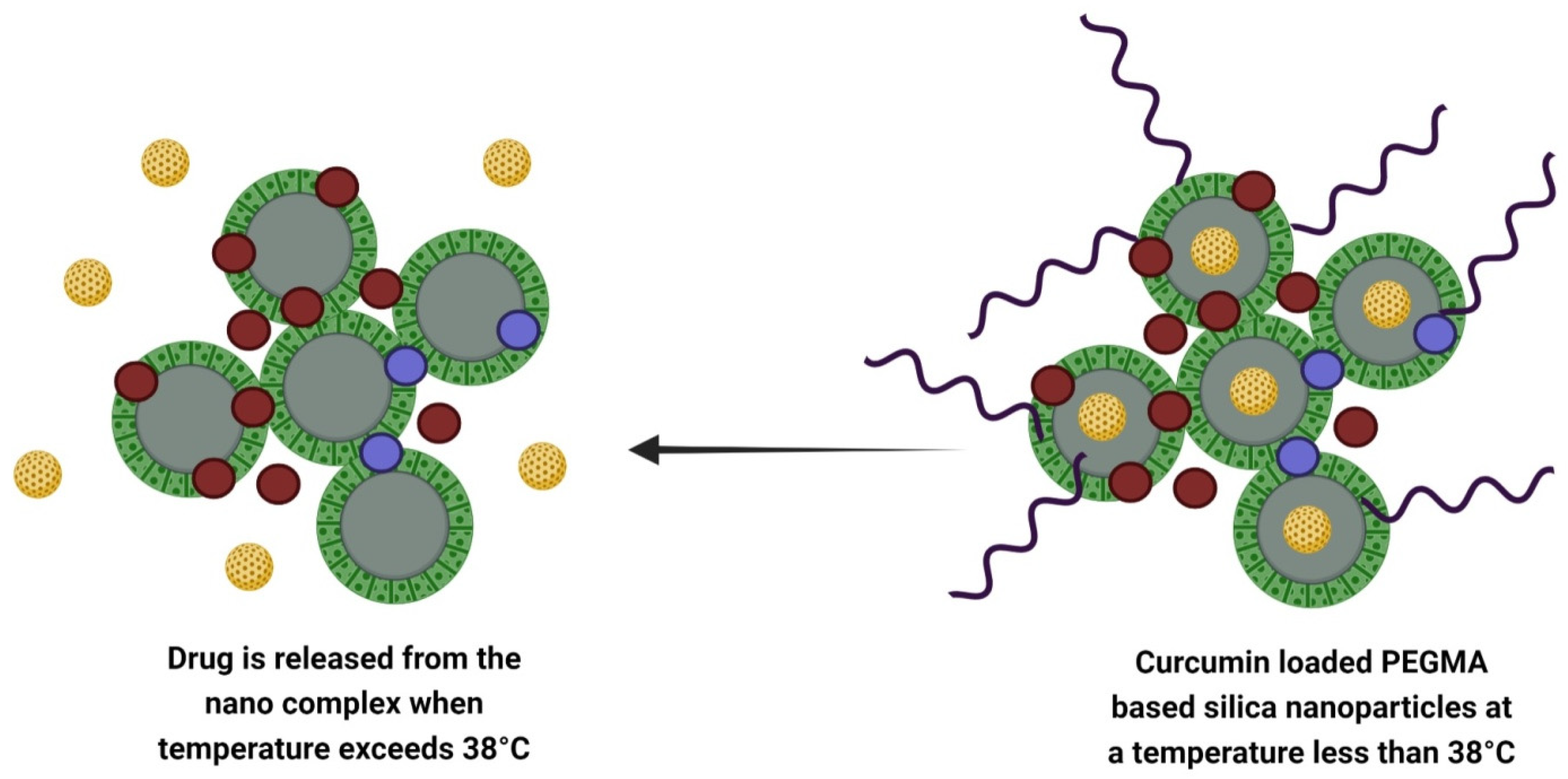
Lo et al. designed a temperature-controlled and dual imaging drug carrier that prevents Zika virus infection when loaded on curcumin (
Figure 2). High fever in Zika infection is used as the basis to design a carrier that controls the release of drugs at a suitable temperature and which is sensitive to rising body temperature to release the drug if symptoms occur. To raise the biocompatibility and solubility of the curcumin, it was loaded into MSNs doped with PEGMA—temperature-responsive polymers and phosphorescent metal ions—i.e., Gd
3+ and Eu
3+. The experimental work was divided into two parts. Firstly, MSNs were synthesized with a temperature-controlled release feature and dual-imaging function with final loading through curcumin. Once formed, the dual imaging function (MSN-EuGd) can be achieved by calcination for pattern removal. Curcumin was loaded after surface modification. Secondly, curcumin-loaded mesoporous silica nanoparticle composite was compared with free curcumin for antivirus capability. Finally, the drug carrier with temperature-controlled features and dual imaging function was synthesized for inhibition of the Zika virus
[15].
Figure 2. Design of curcumin-loaded temperature controlled dual imaging drug carrier system.
The PEGMA surface-modified EuGd-based mesoporous silica nanoparticles act as a temperature-controlled gatekeeper. When the temperature rises above 39 °C, the PEGMA turns into a coil that leads to the opening of the gate. Then, curcumin loads into the MSN pores. On another hand, when the temperature drops below 39 °C, the PEGMA becomes globular in shape over drug-loaded nanoparticles and closes the gate
[15].
4. Application in Food Packaging
Recently, packaging materials with advanced technology have become a topic of great scientific and practical interest
[16][17]. Formal regulations and public tendencies encourage the exploration of biodegradable substitutes for fossil fuel packaging, the extent of which causes widespread damage to the environment. Furthermore, modern packaging materials are predicted to out-perform passive packaging by protecting the enclosed product and increasing its quality and storage. Substances that can inhibit oxygen, absorb moisture, provide antimicrobial protection, control carbon dioxide, absorb ethylene, and neutralize undesirable flavors are in high demand
[18][19]. Klein et al. developed stable antioxidant-antibacterial films via in situ bonds of silica nanoparticle precursors (SNP) with covalently linked curcumin or benzoic acid on polyvinyl alcohol (PVA). The designed films had exceptional antioxidant and antibacterial effects and improved hydrophobicity in protection against undesirable moisture. The growth of the
Listeria innocua as a foodborne human pathogen was completely inhibited via PVA-SNP-ba films. The bacterium was reduced by 2.5 log using curcumin-loaded PVA-silica nanoparticles. Furthermore, the curcumin-loaded PVA-silica nanoparticles-based film displayed a 16 mM/g antioxidant effect as compared to 14.6 mM/g of PVA-silica nanoparticles-ba. Such an approach as the one described could be used as a scaffold for the fabrication of PVA-based packaging materials as an active alternative to silica. Thus, the safe entrapment of bioactive candidates with such biodegradable films could be valuable in both medicine and food industries
[18].
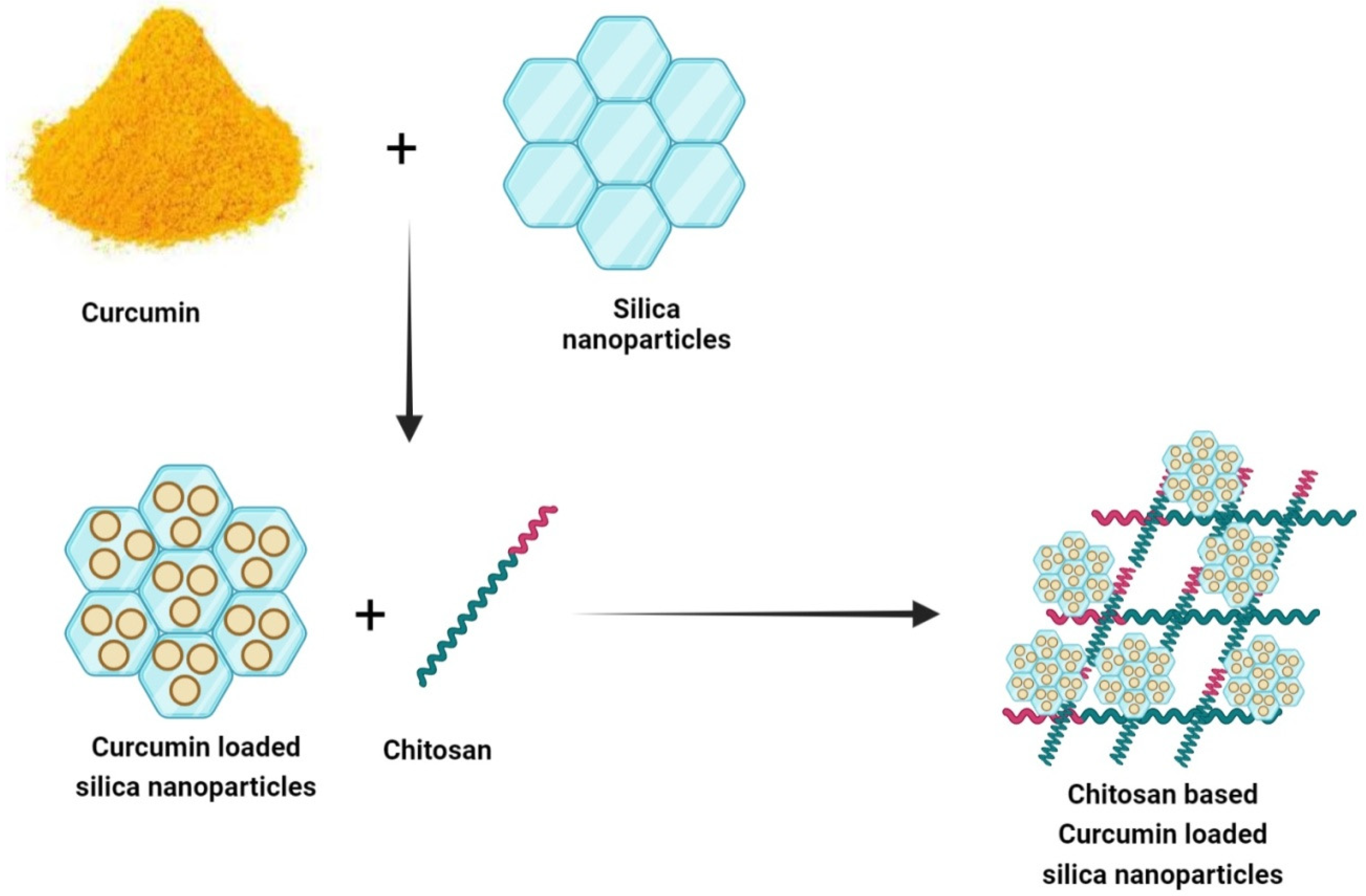
In another study, to improve the functional properties of pure chitosan film, curcumin-loaded mesoporous silica nanoparticles (SBA-15) were inserted into chitosan (CS) film. Curcumin was loaded into SBA-15 (SBA-15-Cur) via the Rotator technique. The CS/SBA-15-Cur nanocomposite film was prepared by solvent casting (
Figure 3). The SBA-15-Cur nanofiller was added to improve the mechanical properties of the nanocomposite film. The CS/SBA-15-Cur film showed effective antibacterial actions against
E. coli and
S. aureus. These results suggest that the CS/SBA-15-Cur nanocomposite film can be a promising material for food packaging
[6].
Figure 3. Preparation of CS/SBA-15/Cur bio-nanocomposite film.
This entry is adapted from the peer-reviewed paper 10.3390/nano12162848