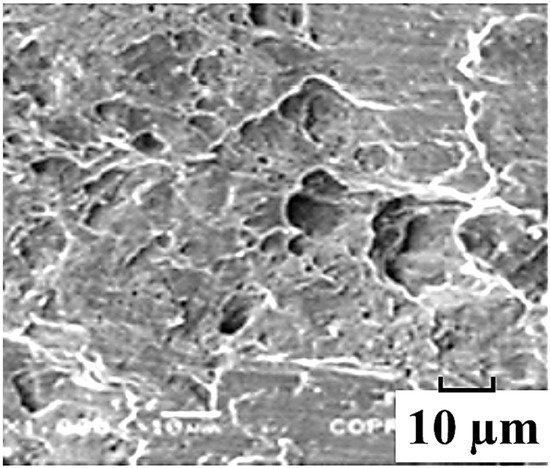
The two major corrosion mechanisms encountered during stress corrosion of carbon steels in a near-neutral pH environment are anodic dissolution and hydrogen embrittlement. Anodic dissolution, also known as metal dissolution, is a process in which the metal is dissolved from the anodic site during corrosion process, and gases are released from the cathodic site and at the metal surface. This in turn reduces the wall strength and may lead to premature pipeline failure. Hydrogen embrittlement can be defined as the reduction of a metal’s tensile strength and ductility due to diffusion of hydrogen atoms into the metal’s crystalline lattices during a corrosion process. It causes premature brittle fracture of normally ductile metals under applied stress usually less than the yield strength of the metal.
1. Introduction
Pipelines are intended to transport various types of fluids (liquid or gas), mixtures of fluids, solids, fluid–solid mixtures, or capsules (freight-laden vessels or vehicles moved by fluids through a pipe) [
1]. They are considered to be a safe, effective and economic means of oil and gas transportation across the globe and have a good safety record [
2,
3]. However, they can suffer from corrosion.
Corrosion is defined as the destruction or deterioration of a metal that results from a reaction with its environment [
4,
5,
6]. Corrosion in both offshore and onshore pipelines is a natural occurring phenomenon and is regarded as one of the major causes of pipeline failures [
2,
4], with corrosion damage accounting for about 20–40% of recorded pipeline failures and incidents [
7]. In 2013, the National Association of Corrosion Engineers (NACE) estimated that the global cost of corrosion was USD 2.5 trillion, which is equivalent to 3.4% of the global gross domestic product (GDP) [
8].
When pipelines are under stress, for example, from internal fluid movement or other outside forces, and are in the presence of a corrosive environment, they can be susceptible to stress corrosion, commonly known as stress corrosion cracking (SCC). Stress corrosion cracking is caused by the combined action of simultaneous mechanical stress and a specific corrosive media that a metal or alloy is susceptible to [
6,
9,
10]. For SCC to occur on a metal or alloy surface, the following three conditions must be met simultaneously: specific conditions to promote crack-propagation must be present; the metallurgy of the material must be susceptible to SCC; and there must be an applied tensile or static stress that exceeds a threshold value [
10,
11,
12]. This tensile stress can originate from centrifugal forces, external loads, temperature variations or internal stresses induced by heat treatment. Stress may also result from locked-in residual stress from fabrication or welding [
6,
9,
11]. SCC cracks normally propagate trans-granularly and/or inter-granularly or may be branched depending on the type of metal/corrosive media combination [
9,
10,
11,
12]. In the trans-granular mode of cracking, the crack advances without a defined preference for the grain boundaries, while in the intergranular mode, the crack proceeds along the grain boundaries.
Generally, two types of SCC have been outlined for pipeline carbon steels in the literature: high pH SCC and near-neutral pH SCC [
13,
14,
15,
16,
17] High pH SCC is characterised by an intergranular mode of cracking and has been investigated in concentrated carbonate/bicarbonate environments with pH values higher than 9 [
13,
14,
15,
16,
17]. Conversely, near-neutral SCC (pH = 6–8) is characterised by a trans-granular cracking mode and is associated with dilute carbonate/bicarbonate medium [
13,
14,
15,
16,
17].
This entry is adapted from the peer-reviewed paper 10.3390/met12081397