Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Wound healing is a recovering process of damaged tissues by replacing dysfunctional injured cellular structures. Wounds occur as a result of accidental or surgical trauma and from a variety of medical conditions. This wound often causes pain, inflammation, and loss of function, which affects a patient’s life and financial costs.
- wound healing
- natural compounds
- bioactivity
- anti-inflammation
1. Introduction
Wounds occur as a result of accidental or surgical trauma and from a variety of medical conditions. This wound often causes pain, inflammation, and loss of function, which affects a patient’s life and financial costs [1]. Wounds are classified as acute wounds or chronic wounds. Wound healing is a complex process of replacing damaged and dysfunctional cellular structures and tissue layers [2]. Acute wounds go through stages of healing, and signs of healing are well-defined within four weeks. Chronic wounds do not undergo normal progression through the healing phases, and healing is not apparent within four weeks. It can be said that the wound healing process depends on factors at the wound site, systemic mediators, type of injury, or any underlying disease [3]. Wound treatment is mainly performed by strategies such as physical closure of the wound margin, sutures, and dressings. When the wound is inaccessible, leave the wound open and let the damaged area clear itself and fill with connective tissue, and the healing process occurs sequentially through phases.
Natural compounds have been used for thousands of years to treat wounds. Natural compounds are found in many plants and animals, which are an abundantly available source for wound treatment. They have proven effective in healing through Chinese and Indian traditional medicines. Due to a vast number of natural compounds, reviews of those compounds would benefit readers and researchers in systematically finding interesting compounds and developing new products for wound healing treatment. Previously, many review papers discussed natural compounds for wound healing treatment [1,4,5,6,7,8,9,10,11,12]. For example, Ryall and colleagues discussed current advancements in skin delivery of natural bioactive compounds for wound management (e.g., turmeric, green tea, honey, garlic, aloe vera, etc.) [4]. Vitale et al. focused on medicinal plants’ phytochemistry and biological activity in wound healing [5]. Ataide and colleagues discussed the activities of pro-wound healing compounds and their mode of action [7]. Dumitru et al. discussed bee products for wound healing treatment [13]. Fana et al. reviewed natural wound healing compounds in traditional Iranian medicine [11]. Those reviews provided many natural compounds for wound healing treatment. However, they only gave tables or lists of natural compounds regarding categories, bioactivities, and mode of action. Those reviews lack discussion on which phase of wound healing natural compounds are affected. Readers might find difficulty when they want to search for information on interesting compounds (wound healing phase, category, chemical formula, mechanism, etc.).
2. The Process of Wound Healing
Wound healing is a process consisting of four phases: hemostasis, inflammation, proliferation, and remodeling. Illustration of the wound healing process is shown in Figure 1.
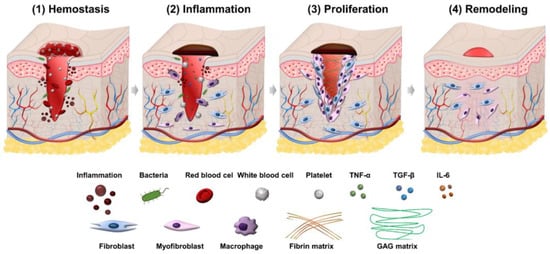
Figure 1. Illustration of four phases in the wound healing process.
2.1. Hemostasis Phase
Wound healing first begins with hemostasis. The lymphatic vessels are injured in this phase, and blood flows out to remove microorganisms and antigens [14]. The body will activate different clotting cascades and thrombocytes to agglomerate by exposed collagen. At the same time, platelets activate vasoconstriction to reduce blood loss and fill tissue gaps in injured vessels with blood clots containing cytokines and growth factors [15]. The clot contains the molecules fibrin, fibronectin, vitronectin, and thrombospondin, which form a temporary matrix as a scaffolding structure for the migration of leukocytes, keratinocytes, fibroblasts, and endothelial cells, and it is a reservoir of growth factors that stabilize blood clots and avoid bleeding.
2.2. Inflammation Phase
The second phase of wound healing is inflammation which focuses on cleaning the wound and preparing for new tissue formation in the wound. This stage has the appearance of neutrophils and lasts about 2–5 days from when the wound becomes infected. Neutrophils can phagocytize and secrete proteases (elastase, cathepsin G, proteinase 3) that help destroy bacteria in the wound and deco remove debris. Neutrophils also release mediators (TNF-α, IL-1 and IL-6) to amplify the inflammatory response, stimulating VEGF and IL-8 to respond to repair during wound healing [16]. The macrophage process then supports the ongoing process by phagocytosis of the debris and secretion of growth factors, chemokines, and cytokines [17]. Macrophages promote and address inflammation, eliminate apoptotic, and support cell proliferation and tissue recovery after injury [18]. In the inflammatory phase, there are often symptoms of edema, erythema and pain.
2.3. Proliferation Phase
The proliferation phase is the most important phase of the wound healing process and lasts from 6 to 21 days. During the proliferation phase of wound healing, the wound is healed with fresh collagen and extracellular matrix tissue. After that, the wound shrinks as new tissues develop. A new network of blood vessels must be created for granulation tissues to remain healthy and receive an adequate supply of nutrients and oxygen. The modulation of fibroblasts toward myofibroblasts promotes the formation of granulation tissue. The myofibroblasts are characterized by the capacity to produce force and synthesize extracellular matrix components that allow the contraction of granulation tissue [19]. By gripping the wound boundaries and pulling them together, myofibroblasts use a technique akin to that of smooth muscle cells to close the wound. In the initial stages of wound healing, granulation tissue appears pink or red and has an uneven texture. Furthermore, healthy granulation tissue is clot-resistant [20,21]. Dark granulation tissue may be brought on by an infection, ischemia, or insufficient perfusion. Near the conclusion of the proliferation phase, epithelial cells resurface the wound. Keeping wounds moist accelerates epithelialization. Epithelialization occurs when occlusive or semi-occlusive dressings are applied within 48 h after the injury. This is because adequate tissue humidity is maintained. One accomplishment of the proliferation phase is replacing the temporary fibrin matrix with a new matrix made of collagen fibers, proteoglycans, and fibronectin to restore the structure and function of tissues. Another crucial stage of healing is angiogenesis, or the ingrowth of new capillaries to replace previously damaged vessels and restore circulation. The creation of granulation tissue and epithelialization are other important phenomena in this healing period. In the proliferation phase of healing, fibroblasts are the most important cells [22,23]. For fibroblasts to migrate in the extracellular matrix, they must first recognize and interact with particular matrix components. Fibroblasts in the normal dermis are usually dormant and sparsely scattered, but they are active and plentiful in the provisional matrix wound site and granulation tissue [24,25]. Their migration and aggregation in the wound site necessitate morphological changes and the production and secretion of proteases to clear a passage from the ECM into the wound site. The chemotactic growth factors, cytokines, and chemokines concentration gradient, as well as the alignment of the fibrils in the ECM and provisional matrix, control the direction of fibroblast migration. Rather than crossing these fibrils, fibroblasts prefer to move along them [26,27]. To help them move through the matrix, fibroblasts produce proteolytic enzymes on a local level. Collagenase (MMP-1), gelatinases (MMP-2 and MMP-9) that destroy gelatin substrates, and stromelysin (MMP-3), which has various protein substrates in the ECM, are three kinds of MMPs released by fibroblasts [28,29]. After migrating into the matrix, fibroblasts change shape, settle down, and begin to proliferate and generate granulation tissue components such as collagen, elastin, and proteoglycans. Fibroblasts connect to the provisional fibrin matrix cables and begin producing collagen [19,30]. Type III collagen, like other extracellular matrix proteins and proteoglycans, is generated in high amounts at first [31]. Collagen mRNA is connected to polyribosomes on the endoplasmic reticulum, where new collagen chains are formed after transcription and processing. A crucial stage in this process involves proline and lysine residue hydroxylation.
2.4. Remodeling Phase
Closure of acute and chronic wounds is regarded as the wound healing endpoint in most clinical settings, yet wounds can continue to undergo remodeling or tissue maturation for months or even years [32,33]. This final stage of wound healing decides whether scarring will occur and whether the wound will reoccur. Regression of the neo vasculature, a periodic deposition to the ECM, and subsequent reconstruction of granulation tissue to scar tissue are all part of the remodeling phase [26]. Collagen III makes up the majority of granulation tissue, which is gradually replaced by the stronger collagen I as the wound heals. This occurs due to simultaneous collagen I production and collagen III lysis, followed by ECM remodeling [34]. In the remodeling phase, scar tissues are created, and it might take several months or years to complete, depending on the severity and location of the wound, and used therapeutic procedures. During this time, the new tissue gradually gets stronger and more flexible. Elasticity and tensile strength of the skin are both getting stronger because of collagen synthesis. After re-epithelialization, macrophages regain their phagocytic phenotype. Excessed cells and matrix no longer required for wound healing are phagocytosed by Mreg or M2c-like macrophages [24].
This entry is adapted from the peer-reviewed paper 10.3390/ijms23179573
This entry is offline, you can click here to edit this entry!
