Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
Alzheimer’s disease (AD) is the most common age-related dementia. The alteration in metabolic characteristics determines the prognosis. Patients at risk show reduced glucose uptake in the brain. Additionally, type 2 diabetes mellitus increases the risk of AD with increasing age. Therefore, changes in glucose uptake in the cerebral cortex may predict the histopathological diagnosis of AD.
- Alzheimer’s disease
- diabetes
- glycolysis
1. Introduction
Alzheimer’s disease (AD), the most generic form of dementia, is an irreversible, progressive brain disorder that destroys neuronal cells. AD is the fifth leading cause of death for people aged sixty-five and older [1]. Scientists do not yet fully understand the cause of this disease, which is likely to involve several factors and can affect each person differently. Health care providers often fail to diagnose AD early; thus, researchers are currently working on a diagnostic framework in which AD onset can be detected based on biological changes in the brain and body before any symptoms appear [2]. Early AD identification remains challenging as the conventional biomarkers for AD can overlap with the classical aging process. Scientists have grouped the AD biomarkers into the ATN framework, where A is amyloid, T is phosphorylated tau, and N is neurodegeneration [3]. Markers such as amyloid-β (Aβ) plaques and tau tangles are well-known indicators of AD; however, these proteins are often present at a higher physiological level in older adults [4,5,6].
Recent studies suggest that dysfunctional glucose metabolism is often found in AD brains. An aged-matched comparison between regular and AD brains showed reduced glucose utilization, evidenced in APP (AD model) mice [7]. Thus, glucose utilization could be an early important imaging marker for AD detection. Under normal physiological conditions, brain cells use a relatively higher percentage of glucose for their function and energy source [8]. Alterations in cerebral glucose metabolic rate and glucose consumption are reflected in the synaptic excitability and neuronal activity [9]. In the AD brain, a lesser extent of glucose utilization was detected by positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) as a tracer [10]. Specifically, a reduction in glucose consumption at the hippocampal and posterior cingulate was observed in the early AD stages [11,12,13]. Later, in the advanced stages, glucose consumption reduces at the temporal-parietal cortex and the frontal and occipital cortices [12]. The decline in glucose metabolism is, moreover, correlative with synaptic density and function [14,15], suggesting that cognitive impairment has a connection to glucose consumption in the brain. The reduction in glucose consumption is directly linked with type 2 diabetes mellitus (T2DM), characterized by insulin resistance and chronic systemic inflammation. Now scientists are concentrating on the relationship between T2DM and the development of AD syndrome in the later stages of life.
2. Cerebral Glucose Metabolism
AD patients show reduced glucose metabolism in the brain. Studies have shown that improved glucose uptake and consumption have neuroprotective effects, including increased proteostasis [16]. Dysregulated glycolytic enzymes reduce glucose utilization and, alternatively, by increasing the protein glycosylation process, induce age-related neurodegeneration [17,18]. Moreover, cerebral glucose uptake in the aged brain reduces due to the reduction in the expression of the essential glucose transporters, GLUTs. For example, neuronal GLUT3 and GLUT4 expression are significantly reduced due to the lower insulin responsiveness in the T2DM brain. In addition, the glycolytic enzyme triose-phosphate isomerase (TPI) plays a crucial role in neurological dysfunction due to mutations [19]. Dysfunctional TPI is responsible for reducing the NADH content. Insufficient NADH generation reflects mitochondrial hypometabolism in TgAD mice [20]. Alternatively, inactivated TPI is also responsible for the accumulation of dihydroxyacetone phosphate (DHAP), which decomposes to methylglyoxal (MG) and is responsible for the accumulation of advanced glycation end-products (AGEs) in AD patients’ brains (Figure 1A) [21].
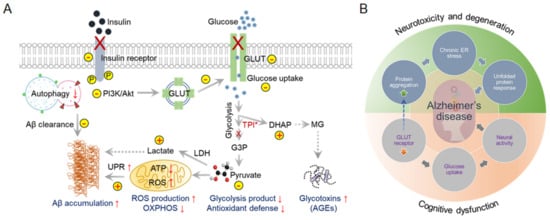
Figure 1. Dysfunctional cerebral glucose metabolism is a crucial factor in AD development. Glucose catabolism in AD is directly connected to further negative consequences in AD syndrome development. (A) Schematic representation of defective glucose metabolism in AD and various outcomes. ↑ Arrows indicate upregulation, and ↓ arrows indicate downregulation. (B) Glucose metabolism forms the bridge between neurotoxicity and cognitive dysfunction in AD. This interconnected Venn diagram describes the scenarios in which AD-related toxicity and cognitive dysfunction appear during AD onset and progression.
In addition to the TPI abnormalities, the lactate dehydrogenase (LDH) activity level increases in aged brains. LDH reduces pyruvate concentration by catalyzing pyruvate to lactate in neurons [22,23,24]. A reduction in pyruvate levels alters the antioxidant defense mechanism and oxidative phosphorylation (OXPHOS) in mitochondria [25,26], triggering oxidative stress and a misfolding protein response. Abnormal glycolytic pathways in the brain could be an early hallmark of AD (Figure 1B). Impairment of the default glucose catabolic process can lead to Aβ deposition or Tau protein phosphorylation [27]. Thus, targeting metabolic reprogramming in neurons is a novel approach for AD intervention and treatment.
3. Monitoring Glucose Uptake and Metabolism in AD
The human brain uses about 20% to 25% of the total glucose utilization in the body to perform the synaptic activity. Principally, the alterations in glucose utilization practically hinder natural cellular functions, including synaptic functions in the brain. Typically, insulin receptors in brain cells control the process of glucose consumption and metabolism. Thus, the development of insulin resistance significantly raises the risk of AD [28,29]; additionally, T2DM increases the risk of AD by 50% [30,31].
In the AD brain, a lesser extent of glucose utilization was detected by positron emission tomography (PET) using 18F-fluorodeoxyglucose (FDG) as a tracer, which is a radiolabeled sugar (glucose) molecule (Figure 2). Imaging with 18F-FDG PET was used to determine the sites of abnormal glucose metabolism [32]. This detection method is now extensively used to locate tumors and sites of recurrent disease in cancer patients (ClinicalTrials.gov Identifier: NCT00207298). The use of 18F-FDG PET imaging is growing because a decrease in glucose utilization correlates with the appearance of cognitive symptoms in AD [33]. Thus, 18F-FDG PET is now considered a practical and independent biomarker for examining the metabolism changes in the pre-symptomatic phase of AD [34]. However, the use of 18F-FDG PET is still limited due to its high cost and limited availability in general clinics around the globe. Moreover, the radioactive material is unsuitable for repeated patient measurements, despite its high specificity and sensitivity.
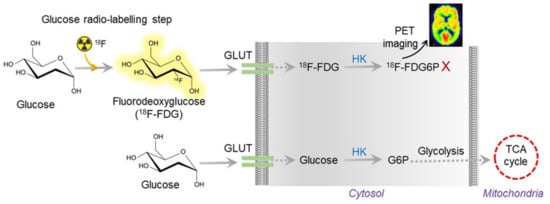
Figure 2. Overview of 18F-FDG PET working mechanism. The radioactive FDG is converted to FDG6P, which can be measured and quantified by PET imaging. In AD, glucose uptake is vastly reduced due to insulin resistance. Thus, the radioactive tracer does not show up in AD brain imaging.
As an alternative, magnetic resonance imaging (MRI) is receiving attention for monitoring sugars in tumor samples [35]. MRI scans can identify brain malformations connected with mild cognitive impairment (MCI) and predict which patients with MCI may eventually develop AD. Nontoxic glucose can be sensed and imaged by dynamic glucose-enhanced (DGE) MRI. DEG-MRI can identify the brain’s glucose distribution, transport, uptake, and metabolic rate [36]. Recent animal studies have demonstrated that d-glucose is a biodegradable MRI contrast agent for imaging glucose uptake in tumors [35,37].
Additionally, d-glucose can cross the blood-brain barrier (BBB), making DGE-MRI feasible for studying glucose uptake and metabolism in the human brain [38]. Experiments involving WT mice and age-matched APP/PS1 transgenic AD mice demonstrated a notable reduction in signal intensity for d-glucose uptake in the brain parenchyma and CSF. Reduced glucose signals could result from impaired glucose uptake in the AD brain due to reduced GLUT1 and GLUT3 expression at BBB [7]. Recently, Huang et al. modified the DGE-MRI to detect D-glucose delivery in the brain [7] and identified altered glucose uptake and clearance in young AD mice before the emergence of amyloid plaques. Another widely explored imaging technique in cancer metabolism is chemical exchange saturation transfer (CEST) MRI [39,40]. Scientists used CEST-MRI to detect 2-deoxy-D-glucose (2DG) uptake in AD mouse (APP23 model) brains in different regions of the brain [41]. CEST technique can distinguish the decline in 2DG brain uptake in 20-month-old APP23 mice compared to WT mice. The brain glucose metabolic activity differences can be detected within a few minutes of injecting 2DG into the cortex area [42]. It is necessary to employ a 3T clinical field strength and use nontoxic sugars to expedite the conversion of DEG-MRI or CEST-MRI to clinical AD diagnosis.
This entry is adapted from the peer-reviewed paper 10.3390/ijms23179540
This entry is offline, you can click here to edit this entry!
