Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Biochemistry & Molecular Biology
La prevalenza dell'obesità è aumentata costantemente in tutto il mondo negli ultimi tre decenni. I polifenoli possono essere considerati nutraceutici e integratori alimentari consigliati per diverse sindromi. I polifenoli sono una classe di sostanze fitochimiche presenti in natura, alcune delle quali hanno dimostrato di modulare le vie fisiologiche e molecolari coinvolte nel metabolismo energetico. I polifenoli potrebbero agire nella stimolazione della β-ossidazione, nell'inibizione della differenziazione degli adipociti, nel contrastare lo stress ossidativo, ecc.
- polyphenols
- obesity
- antioxidants
- oxidative stress
1. Azioni di alcuni polifenoli
La curcumina ( Figura 4 ) è il polifenolo più bioattivo nella Curcuma longa , una pianta solitamente consumata come spezia in India e in altri stati asiatici. È stato utilizzato per migliaia di anni in una medicina dell'Ayurveda, che significa "scienza della lunga vita", e le prime notizie sulla curcuma come medicinale utile sono datate 3000 aC La curcumina esercita diverse funzioni biologiche, tra cui antiossidante, antinfiammatoria e antiangiogenesi, in diversi organi, compreso il tessuto adiposo [ 58 , 59 ]. Esistono prove sostanziali sull'efficacia della curcumina nello stimolare la β-ossidazione, nell'inibire la sintesi degli acidi grassi e nel ridurre l'accumulo di grasso [ 60 , 61 ].
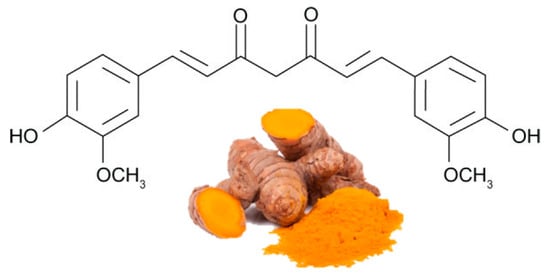
Figura 4. Le principali fonti di curcumina e la sua struttura chimica (forma cheto).
Numerose ricerche precliniche e cliniche hanno dimostrato l'effetto benefico della curcumina nell'attenuare l'aumento di peso corporeo, nel migliorare la sensibilità all'insulina e nella prevenzione dello sviluppo del diabete [ 62 , 63 , 64 ].
A differenza degli studi sugli effetti della curcumina nelle cellule o negli animali, gli studi sui soggetti obesi sono limitati. Il primo studio clinico che utilizza la curcumina per il trattamento dell'obesità è stato condotto da Mohammadi et al. [ 65 ]. In questo studio, i soggetti obesi sono stati trattati con una formulazione commerciale di curcumina (1 g/die) integrata con un potenziatore della biodisponibilità, la piperina, per un mese. Sebbene non ci siano state variazioni di peso, BMI o grasso corporeo, i livelli sierici di trigliceridi sono stati significativamente ridotti dopo il trattamento con curcumina, indicando il miglioramento delle azioni dell'insulina [ 65 ] ( Tabella 1 ). In un altro studio randomizzato, Ganjali e Sahebkar hanno dimostrato che il trattamento di 30 giorni con curcumina (500 mg/die) riduce i livelli sierici delle citochine infiammatorie IL-1β e IL-4 degli individui obesi.66 ], indicando l'attività antinfiammatoria della curcumina nella terapia dell'obesità. Inoltre, l'integrazione orale di curcumina (1 g/die per 30 giorni) è stata efficace nel ridurre il carico di stress ossidativo negli individui obesi [ 67 ]. Uno studio di controllo randomizzato condotto da Di Pierro et al. [ 68 ], tra le persone in sovrappeso affette da sindrome metabolica, hanno mostrato la capacità della curcumina di ridurre il peso e il tessuto adiposo omentale. La supplementazione di curcumina ha avuto effetti benefici sull'indice di massa corporea, sulla circonferenza della vita, sulla circonferenza dell'anca, sui livelli di lipoproteine ad alta densità e sul rapporto trigliceridi/lipoproteine ad alta densità nelle adolescenti in sovrappeso e obese [ 69 ] ( Tabella 1 ).
Tabella 1. Azioni anti-obesità di curcumina, quercetina e resveratrolo.
| POLIFENOLO | Prove umane | Effetti | Riferimenti * |
|---|---|---|---|
| Curcumina | Studio randomizzato, in doppio cieco, controllato con placebo, crossover 30 soggetti obesi 30 giorni di trattamento con curcumina (1 g/giorno) |
= Peso, BMI, % di grasso corporeo ↓ TG |
65 |
| Studio crossover randomizzato su 30 soggetti obesi Trattamento di 30 giorni con curcumina 1 g/die per 4 settimane |
↓ Livelli sierici di VEGF ↓ IL-1 β e IL-4 |
66 | |
| Studio randomizzato in doppio cieco, controllato con placebo, cross-over su 30 soggetti obesi Integrazione di curcumina (1 g/die per 30 giorni) |
↓ Carico di stress ossidativo | 67 | |
| Studio randomizzato e controllato. 44 soggetti in sovrappeso 30 giorni con curcumina |
↑ Perdita di peso ↓↓ % di grasso corporeo ↑ Riduzione del giro vita, ↓↓ Circonferenza dell'anca ↓↓ BMI |
68 | |
| Studio clinico randomizzato controllato con placebo 60 Adolescenti di sesso femminile in sovrappeso e obese. Compressa da 500 mg al giorno (95% di curcumina) |
↓ BMI ↓ Circonferenza vita ↓ Circonferenza fianchi ↓ LDL |
69 | |
| Quercetina | Studio crossover in doppio cieco su soggetti obesi in sovrappeso 150 mg/die di quercetina per 8 settimane |
↓ Circonferenza vita ↓ Pressione sistolica postprandiale ↓ TG |
76 |
| Double-blinded, placebo-controlled cross-over trial 70 Overweight-to-obese subjects 162 mg/d quercetin 6-week treatment periods |
↓ Ambulatory blood pressure | 77 | |
| Resveratrol | Randomized double-blind crossover study 11 obese men 150 mg/day resveratrol for 30 days |
↓ Insulin, Plasma Fatty Acids, ↓ TG, Glucose, Leptin |
85 |
| 11 healthy obese men 30 days (150 mg resveratrol/day) |
↓ Adipocyte size | 86 |
* Anti-obesity human studies. IL: InterLeukin; BMI: Body Mass Index; TG: TriGlyceride; LDL: High Density Lipoprotein; VEGF: Vascular Endothelial Growth Factor.
Overall, these research studies show that curcumin applies anti-obesity and anti-inflammatory actions in part through adipose tissue, by decreasing adiposity, lipid storage, and enhancing lipid oxidation [70]. Although curcumin has been used in clinical trials, its multifaceted pharmacological nature, its pharmacokinetics, and its side effects in obesity therapy need to be carefully investigated. The recommended maximum daily usage of curcumin is 1 mg/kg body weight by a joint report of the World Health Organization (WHO) and the Food and Agriculture Organization [71]. Moreover, a few studies showed that the chronic use of curcumin can cause some upset [72]. Recent studies suggest that the metabolic effects of curcumin are linked to changes in the gut microbiota. However, research into curcumin continues to provide novel insights into metabolic regulation that may ultimately translate into effective therapy [73].
Quercetin (Figure 5) is one of the major flavonoids, and one of the most potent antioxidants of plant origin. It is found in many foods, including vegetables, such as onions, garlic, and ginger, fruit, such as apples, and wine. Although many in vitro and in vivo studies focused on the beneficial effects of quercetin in obesity, there are only a limited number of human studies and clinical trials that have been performed to evaluate the effects of quercetin on obesity treatment [70,74,75]. A study evaluated the effects of taking quercetin in overweight obese subjects with various apolipoprotein E genotypes; the quercetin (150 mg/day/subject) decreased the waist circumference and triacylglycerol concentration [76]. Quercetin supplementation improved some risk factors of cardiovascular disease, yet exerted slightly pro-inflammatory effects [76]. One hundred and sixty-two milligrams per day quercetin supplementation lowers ambulatory blood pressure in overweight-to-obese patients, suggesting a cardio protective effect of this polyphenol [77] (Table 1).
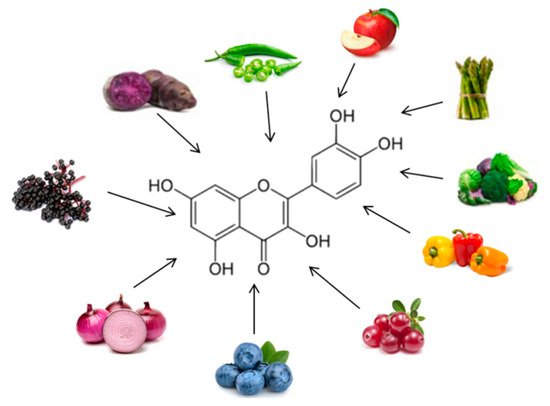
Figure 5. Chemical structure and major sources of quercetin.
Although quercetin suppressed oxidative stress in obese animal models, Shanely et al. reported that quercetin has no effect on oxidative stress and antioxidant (500 or 1000 mg/day/subject for 12 weeks) in obese subjects [78].
Currently, a clinical trial that is still under phase II stage investigation, is investigating whether quercetin changes the absorption of glucose by the body in obese subjects [79]. Future research needs to further investigate the bioactive effects and bioavailability of quercetin.
Resveratrol (RE) (Figure 6) is a polyphenolic phytoalexin found in over 70 plant species and is highly concentrated in the skin of red grapes. Tea, berries, pomegranates, nuts, blueberries, and dark chocolate contain this polyphenol at varying concentrations [80]. RE exhibit a plethora of therapeutic benefits, including anti-inflammatory and antioxidant [81]. RE was discovered to be an activator of sirtuin 1, an important molecular target regulating cellular energy metabolism and mitochondrial homeostasis. An important target of RE is adenosine monophosphate-activated protein kinase (AMPK), suggesting that it can play a role in regulating energy homeostasis; by activating AMPK, RE exerts a lipid-lowering effect. RE has potential anti-obesity effects by inhibiting differentiation and decreasing proliferation of adipocytes, decreasing lipogenesis, and promoting lipolysis and β-oxidation [82].
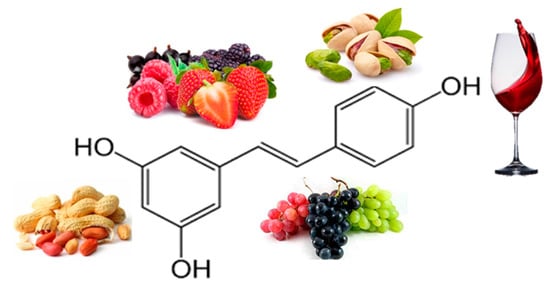
Figure 6. Chemical structure and major sources of resveratrol.
While the effects of RE have been widely studied in animal models [83,84], few clinical studies have been performed, and the results are inconclusive. Moreover, contrary to the substantial preclinical findings of beneficial metabolic effects of RE in an obesity or inflammation setting, the outcome of human clinical trials of resveratrol effects on obesity-related morbidities have been inconsistent. In a cross-over study, Timmers et al. showed that 150 mg/day of RE treatment increased energy expenditure, reduced serum inflammatory markers, and decreased adipose tissue lipolysis and plasma fatty acid and glycerol levels of obese men [85]. In another study, Konings et al. investigated the effects of 30 days RE treatment (150 mg/day) on the adipocyte size and gene expression patterns in obese men. The authors found that RE treatment decreased the size of abdominal subcutaneous adipocytes [86] (Table 1). Some beneficial effects have also been observed in some clinical trials, although many discrepancies and conflicting information exist [87]. Arzola-Paniagua et al. [88] observed not significant decreases in BMI, waist circumference, fat mass, triglycerides, leptin, and leptin/adiponectin ratio in obese individual’s treated with resveratrol therapy. RE treatment did not improve inflammatory status, glucose homeostasis, blood pressure, nor hepatic lipid content in middle-aged men with metabolic syndrome. On the contrary, it significantly increased total cholesterol and LDL cholesterol [89]. RE is used combined with other phytochemicals, too. For example, twelve weeks of combined epigallocatechin-3-gallate and RE supplementation increased mitochondrial capacity and stimulated fat oxidation in obese humans [90].
However, another report showed that high levels of RE supplementation treatment had no effect on energy expenditure, adipose tissue content, and metabolic events. A clinical trial by Poulsen et al. did not seem to support the anti-obesity potential of RE in obese men; no effect was seen on blood pressure, resting energy expenditure, oxidation rates of lipid, ectopic or visceral fat content, or inflammatory and metabolic biomarkers [91]. It has been observed that RE presents a dose–response hormesis in the biological models in which it has been tested, affecting several outcomes with medical and therapeutic significance [92].
Evidence from these limited clinical studies combined with the results from in vitro and animal studies indicate that potential anti-obesity effects of RE may be achieved through dietary supplementation. The differing results could be due to the variable doses selected in the assays and to the different clinical backgrounds of the study subjects. However, the optimal doses and study period for the anti-obesity potential of RE remain to be determined [70,89].
2. Actions of Some Polyphenolic-Food
More numerous are the clinical studies in which the anti-obesity effect of extracts of polyphenolic foods is analyzed.
Many foods, such as apples, blueberries, gooseberries, grape seeds, kiwi, strawberries, green tea, red wine, beer, cacao liquor, chocolate, and cocoa, are rich in polyphenols. The effects of green tea extract (GTE) on obesity have received increasing attention. GTE is rich in polyphenols, including epigallocatechin-gallate (EGCG), epicatechin, epigallocatechin, and epicatechin-gallate: a 2-g bag of green tea contains about 500 mg of catechins [93]. Since the 1990s, green tea is seen as a natural herb that can enhance energy expenditure and fat oxidation, thereby inducing weight loss. In a double-blind study, 46 obese patients received either 379 mg of green tea extract. Three months of GTE supplementation resulted in decreases in body mass index, waist circumference, levels of total cholesterol, low-density cholesterol, and triglyceride. Increases in total antioxidant levels and serum concentrations of zinc and magnesium were also observed. These findings demonstrated that green tea could influence the body’s mineral status, and they showed the beneficial effects of green tea extract supplementation on body mass index, lipid profile, and total antioxidant status in patients with obesity [94]. In another study, 12 weeks of treatment with high-dose GTE resulted in significant weight loss, reduced waist circumference, and a consistent decrease in total cholesterol and LDL plasma levels without any side effects or adverse effects in women with obesity. The antiobesity mechanism of high-dose green tea extract might be associated in part with ghrelin secretion inhibition, leading to increased adiponectin levels [95,96]. Results of randomized, controlled intervention trials have shown that consumption of GTE (270 mg to 1200 mg/day) may reduce body weight and fat [97]. Almost all of the studies conducted with Asian subjects have shown positive results about the anti-obesity effects of tea extract; on the other hand, studies with Caucasian subjects reported mixed results [98]. There are several proposed mechanisms whereby GTE may influence body weight and composition. Green tea also contains caffeine, and there is probably a synergistic action between the different molecules. The fact that an EGCG–caffeine mixture stimulates energy expenditure cannot be completely attributed to its caffeine content because the thermogenic effect of an EGCG–caffeine mixture is greater than that of an equivalent amount of caffeine [99]. The predominating hypothesis is that GTE influences sympathetic nervous system activity, increasing energy expenditure and promoting the oxidation of fat. Other potential mechanisms include modifications in appetite, up-regulation of enzymes involved in hepatic fat oxidation, and decreased nutrient absorption [97].
In conclusion, cellular and animal studies have shown that dietary supplementation with green tea extract is a potentially viable nutritional strategy for the prevention of obesity. However, the efficacy of green tea remains unclear [100]. The low bioavailability of GTE along with potential confounders may have contributed to the inconsistent outcome of human studies [101]. Human trials of course have greater reproducibility difficulties. The discrepancies among the studies employing green tea may be due to the varieties of study designs, the length of study, age and gender of subjects, the ethnicity of subjects, the formulations of green tea supplement, and the presence or absence of weight control factors (i.e., caffeine, exercise, low-caloric diet). While few epidemiological and clinical studies show the health benefits of EGCG on obesity, the mechanisms of its actions are emerging based on the various laboratory data. These mechanisms may be related to certain pathways, such as through the modulations of energy balance, endocrine systems, food intake, lipid and carbohydrate metabolism, the redox status, and activities of different types of cells (i.e., fat, liver, muscle, and beta-pancreatic cells) [102].
Citrus fruits and their juices are natural sources of many bioactive compounds, such as flavonoids and carotenoids, and their properties, such as antioxidant power, derives from these molecules, too [103]. Particularly, grapefruit and bergamot are rich in citrus flavonoids, including naringenin, hesperitin, nobiletin, and tangeretin, which have emerged as potential therapeutics for the treatment of metabolic deregulation [104]. In obese humans, 1/2 grapefruit (49 mg naringenin) three times daily for 8 to 12 weeks reduced body weight and waist circumference [105]. Improvements were observed in circulating lipids, with total cholesterol and low-density lipoprotein significantly decreasing [105]. Grapefruit should be further evaluated in the context of obesity and cardiovascular disease prevention.
Orange juice consumption can promote lower levels of oxidative stress and inflammation due to the antioxidant activity of citrus flavonoids, too. Red orange juice was effective in reducing metabolic markers and inflammatory markers in human subjects [106], but it does not inhibit weight loss. It ameliorated the insulin sensitivity, lipid profile [107], and inflammatory status, as well as contributes nutritionally to the quality of the diet [108]. The authors suggest the consumption of freshly squeezed orange juice, without added sugars, as part of a controlled diet in obese women; the combination with increasing physical activity for maintaining a healthy body weight through healthier food choices could improve the comorbidities related to the obesity [107].
It is opportune encouraging the consumption of whole fruits and replacing packaged fruit juices with fresh fruit juices or plain water, as part of a broader set of dietary strategies to reduce total dietary energy intake in adult populations.
The apple is rich in polyphenols, such as hydroxycinnamic acids, flavonols, dihydrochalcones, and anthocyanidins; the 22% of the dietary phenolic ingested comes from the apples. Daily consumption of apple for 4 weeks increased antioxidant enzymes in an elderly population, likely because of the presence of flavonoids [120]. Apples are associated with decreased risk of obesity in children according to the National Health and Nutrition Examination Survey (2003–2010) [121]. Dried apple can be recommended as a snack for overweight and obese children thanks to its high-fiber and polyphenol content [122]. Thirty-eight overweight or obese children consumed 120 kcal serving per day of either dried apple or a control snack (muffin) for 8 weeks; high-density lipoprotein cholesterol concentration increased within the apple group. Some intervention clinical trials with an apple juice showed a significant reduction in body fat mass but not in body weight, BMI, nor waist circumference [123]. Future research should evaluate the effects of consuming fresh apples that include the peel [122].
The onion is rich in quercetin. A study showed that 12-week of onion extract intake decreased body weight, percentage of body fat, and BMI of 10 female students [124]. The consumption of onion peel extract improves endothelial function in healthy overweight and obese individuals [77,125]. Moreover, Lee et al. demonstrated that onion (100 mg/day/subject) significantly decreased the total body fat, particularly in the percentage of fat in the arm, and decreased the BMI of overweight or obese subjects [126].
Soybean (Glycine max), an East Asian legume, has been approved as a health-promoting food because of its effects in prevention of metabolic disorders, such as hyperlipidemia, cardiovascular diseases, and type 2 diabetes [127,128]. These beneficial effects are supposed to be exerted by constituents, such as unsaturated fats, fiber, the high content of protein, and isoflavones [129]. Isoflavones, i.e., genistein, daidzen, and glycetin, have comparable chemical structures to endogenous estrogens and are believed to interact with intracellular estrogen receptors, which results a possible action in decreases in lipids accumulation and adipose distribution. Both animal and human studies have shown the effect of dietary soy on weight control and prevention of obesity [127]. Studies have revealed that isoflavones are involved in the inhibition of adipogenesis and lipogenesis by interrelating with several transcription factors and upstream signaling molecules [130]. Although the biological mechanisms that cause the actions of soy isoflavones and numerous effects remain unknown, it is noteworthy that isoflavones exhibit pleiotropic properties in the human body to control metabolism and balance, which may potentially inhibit and treat obesity [130]. Various studies show a role of soy in improving health; it causes a decrease in cholesterol, triglycerides, and adiposity, it acts on improving inflammatory response and defense against cancer, osteoporosis, and menopause. Positive soybean actions on inflammation have been reported; soy extract constrain secretion of inflammatory cytokines (TNF-α, MCP-1, and IL-6) and decrease the inflammatory responses in adipocytes [131]. Eighty-seven obese postmenopausal women took soy extract daily, corresponded to 80 mg of isoflavones. After 6 months, serum leptin and TNF-alpha levels declined, and a significant increase of serum levels of adiponectin was detected [132]. Soy-isoflavones studies showed that this extract may reduce body mass index in women, especially in dosages <100 mg/d and in intervention periods of 2–6 mo. In addition,, a trend for reduced BMI after their consumption was observed in Caucasians. Overall, results showed that soy-isoflavones extract may have different impacts on weight status [133]. Overweight human subjects were treated with black soybean extract. This randomized clinical study showed reduction in abdominal fat, cholesterol, triacylglycerol, and LDL levels, with decreasing TNF-α and MCP-1 levels [134]. Studies in obesity models have demonstrated that dietary soy-isoflavones significantly reduced body weight and fat pad weight. It was shown that sterol regulatory element-binding protein 1 SREBP1 transcription factor expression and its other target genes (acetyl-coenzyme A carboxylase, ATP-citrate lyase, and FASN) were reduced by soy isoflavones. Overall, available studies prove that soy-isoflavones decrease adiposity and inflammation, by decreasing lipogenesis, adipogenesis, and improving lipid clearance; they are capable of decreasing oxidative stress and may contribute in metabolic enhancements in obesity.
The search for polyphenolic extracts with antiobesity activity is always active, always new phytochemicals are proposed. Artemisia princeps, the Korean mugwort, is a common edible plant native to Korea, Japan, and China. In Western and African folk medicine, several species of the genus Artemisia are used for their claimed healing properties and for the cure of specific ailments. The early in vitro antiobesity-effects [135] of Artemisia could be due to the presence of active compounds, such as terpenes, steroids, and saponins, and also the active ingredients, such as tannins and flavonoids [136]. Physalis alkekengi, also referred to as ground cherry, is an indigenous herb in Iran and many other regions of Asia such as China. Preliminaries studies have reported presence of several active compounds, including secosteroids called physalins, and flavonoids [137,138]. Single compounds found in Physalis alkekengi should be studied separately to identify the main compound exerting in vivo antiobesity properties [139]. Persimmon (Diospyros kaki) fruit is native to China, and its cultivation extends to other part of East Asia, including Japan, where it is very popular, and it has high proanthocyanidin-type content [140]. I frutti di cachi possiedono alti livelli di fibra alimentare, vitamina C, catechina e gallocatechina [ 141 ]; esercitano effetti benefici sull'obesità inibendo l'adipogenesi e riducendo la sintesi e l'accumulo di lipidi regolando i fattori di trascrizione correlati ai lipidi nel tessuto adiposo bianco dei topi obesi [ 140 ].
This entry is adapted from the peer-reviewed paper 10.3390/ijms21165642
This entry is offline, you can click here to edit this entry!
