Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
The aim of this narrative review is to explore the principles of tendon remodeling under resistance-based exercise in both healthy and pathologic tissues. The associated paper also reviews the biomechanical principles of Achilles tendon loading mechanics which may impact an optimized therapeutic exercise prescription for Achilles tendinopathy.
- exercise therapy
- physical therapy modalities
- rehabilitation
- tendons
- tendinopathy
- mechanotransduction
1. Introduction
Resistance-based therapeutic exercise is the cornerstone of non-surgical Achilles tendinopathy (AT) management [1][2]. Understanding how and why such exercises influence the experience of tendon pain and what factors may govern these effects may aid clinicians and researchers in optimizing therapeutic exercise interventions. Additionally, understanding the impact of therapeutic exercises on tendon function and the changes to the morphological, material, and mechanical properties of the tendon is critical for load management. Despite the prevalence of therapeutic exercise AT management, few works have explored tissue loading optimization for individuals with tendinopathy.
Although passive and relatively inelastic structures [3], tendons facilitate joint movement by transferring forces generated by muscles to the skeleton [4]. Specifically, tendons deform under load to store and return strain energy, making them critical during locomotion [5][6]. Human tendons vary considerably throughout the body in terms of structure [7] and mechanical properties [8], largely attributable to the functional demands of different regional loading environments [3][9][10]. The Achilles is the largest, strongest, and thickest tendon in the body [11], often experiencing forces of 5–7 bodyweights per step during running [12][13][14] and up to 7.3 bodyweights during single-leg hopping [15]. With repetitive or intense loading exceeding physiological limits, individuals may develop AT [16][17].
Achilles tendinopathy is defined as consistent pain in the Achilles tendon coupled with a loss of function associated with mechanical loading [18]. The incidence of AT is approximately 0.2–0.3% in adults (i.e., 2–3 per 1000) [19]. The occurrence substantially increases in runners, with incidences of 5.0–10.9% [20][21][22] in recreational runners and up to 52% in male former elite runners [23]. Achilles tendinopathy can either be classified as insertional AT (symptoms localized 0–2 cm from the distal insertion; 20–25% of Achilles tendon injuries) or midportion AT (symptoms localized 2–7 cm proximal to the insertion; 55–65% of Achilles tendon injuries) [1][24]. Diagnosis of insertional AT can often be confounded by additional pathologies, such as Haglund’s deformity, retrocalcaneal bursitis, and retrocalcaneal exostosis [25]. Given structural and functional differences across the Achilles tendon [26], it is important to distinguish between insertional and midportion AT as treatment option efficacy can differ [1][25]. Achilles tendinopathy can result in substantial localized pain and morphological changes to the tendon leading to deficiencies in material properties and mechanical behaviors [27]. If continuously subjected to the same detrimental loading patterns, the tendon structure can deteriorate further increasing the chance of rupture [28].
It is well established that resistance exercise positively remodels the healthy Achilles tendon [29][30][31][32]. Additionally, therapeutic exercise is consistently touted as a standard non-surgical treatment for AT [1][2], largely independent of muscle contraction type (i.e., concentric, eccentric, or isometric) [33][34][35][36]; however, the mechanism of therapeutic action is still a subject of debate and exploration [36][37][38][39]. Resultingly, much of the clinical research for AT has focused on combining resistance exercises with other treatments as opposed to optimizing the exercise program itself [40][41]. Although aspects of loading optimization have been investigated in healthy persons [42][43][44], the translation and applicability of these principles to individuals with AT has not been reported.
2. Anatomy Tailored for Function
This section provides a brief overview of several major anatomical considerations related to the biomechanics of the Achilles tendon and the triceps surae muscle-tendon unit (MTU).
2.1. Achilles Tendon Homeostasis and Structure
As outlined by Thorpe and Screen [3], the Achilles tendon is composed of approximately 20% cellular material and 80% extracellular matrix (ECM). Approximately 55–70% of the ECM is water, with the remaining portion corresponding primarily to highly organized Type I collagen and to a lesser extent Type III, V, and XI collagen, as well as non-collagenous molecules, such as proteoglycans, which promote ECM organization. The ECM is actively regulated by tendon fibroblasts, also known as tenocytes, which present with an elongated morphology and function primarily to control collagen synthesis. Importantly, tenocytes are mechanosensitive and have several force-sensitive provisions, such as integrins and stretch-activated ion channels, which allow them to modulate tendon collagen and non-collagenous content through cell signaling pathways thereby influencing tendon tissue mechanical properties [45]. Tenocytes are distributed both within and between tendon fascicles, which are a distinct unit amongst the tendon structural hierarchy. Amongst the collagen fibers, tenocytes form a three-dimensional network with cellular extensions expanding into the ECM [46], allowing them to sense substrate strain [47] and communicate these signals to adjacent cells via gap junctions [48] thereby promoting load monitoring throughout the tendon.
2.2. Force Transmission within the Achilles Tendon
Components of the tendon microstructure including collagen, elastin, and tenocytes are generally oriented along the longitudinal axis, resulting in anisotropic behavior and high tensile strength [49][50]. Additionally, the fluid within the tendon gives it viscoelastic properties [49][51]. The Achilles tendon is structured to temporarily store and return large amounts of kinetic energy from primarily tensile loads, some exceeding 9 kN [52][53], which is critical for efficient movement [4][49]. The Achilles tendon also optimizes the force generated by the triceps surae muscles by governing the force-length-velocity relationship [54][55]. On the proximal end, the Achilles tendon is the tendinous continuation of the triceps surae which proceeds to medially rotate until inserting distally on the posterior calcaneus [56][57]. As such, the proximal end of the Achilles tendon is cyclically deformed by the triceps surae muscles, while the distal end is fixed to the calcaneus via the enthesis, which serves to mitigate stresses at the hard-soft tissue interface [58]. The primary loading profile of the Achilles tendon underlines that stress and strain vary across the Achilles, but controlling tensile loading along the longitudinal axis is critical [59][60].
Tissue mechanics at all levels of the tendon hierarchy promote the load tolerance of the Achilles tendon [49]. Briefly, the smallest level of the hierarchy is the tropocollagen molecule, which is the structural unit of collagen fibers, and is composed of three polypeptides forming a triple-helix structure stabilized by hydrogen bonds [61]. Tropocollagen molecules are extensible under tensile loading via helix elongation [62] and lateral molecular order increases when tension is applied [63], possibly indicating alignment with the principal loading direction [49]. Staggered tropocollagen molecules self-assemble to form collagen fibrils [49], which take a mature form when covalently cross-linked through the enzymatic action of lysyl oxidase [64][65]. Cross-links are fundamental to the load-bearing capacity of the fibril [66][67], and cross-link density (or lack thereof) directly influences tendon mechanics by governing intra-fibril sliding [68]. At the fiber level, the collagen fibrils are oriented along the longitudinal axis in a distinct pattern known as ‘crimp’, which contributes to load tolerance as the crimp-pattern straightens near the onset of tensile loading [69]. Additionally, collagen fiber sliding appears crucial to tendon elongation [70]. Collagen fascicles are generally considered continuous throughout the tendon [49] and may act primarily as independent load-bearing structures with negligible lateral force transmission at low strain levels [71]. With that said, work investigating mechanical loading at higher load levels (up to the point of rupture) concluded that both the spiral twisting of the fascicles and sliding within the Achilles tendon considerably improve tissue strength by more evenly distributing stresses across the whole tendon [72]. In sum, features throughout the tendon hierarchy are responsible for global tendon elongation, though testing heterogeneity makes it challenging to isolate relative contributions [49].
2.3. Force Transmission within the Triceps Surae Muscle-Tendon Unit
The triceps surae (i.e., medial gastrocnemius [MG], lateral gastrocnemius [LG], and the soleus [SOL]) is responsible for the majority of plantarflexion force generation which enables locomotion [73][74]. While the uniarticular SOL acts as the main plantar flexor muscle [75], the bi-articular gastrocnemius functions to both flex the knee and contributes to ankle plantarflexion [73]. In addition to function, SOL also differs from gastrocnemii in fiber type [76] and architecture [77][78]. The approximate 6:2:1 physiological cross-sectional area (CSA) relationship of the SOL, MG, and LG [79] indicates that the maximal force-production capacity of the SOL is considerably greater than that of the gastrocnemii as physiological CSA is directly linked to muscle force production [80][81]. Each of the triceps surae muscles insert onto the calcaneus by way of three different ‘subtendons’, which originate from each muscle and represent distinct functional portions of the Achilles tendon [56][57] (Figure 1). Both the subtendons and the fascicles comprising them rotate counterclockwise in the right limb and clockwise on the left, though the extent of rotation varies considerably between individuals [56][82][83]. Further, the fascicles tend to fuse distally creating a more uniform tendon structure [72][82]. The difference in muscular force potentials and subtendon transmission pathway through the Achilles may have implications on the incidence of AT through modifications to tendon mechanical properties [84], strain distribution [60][85][86], and shear generated from subtendon sliding [26][86][87].
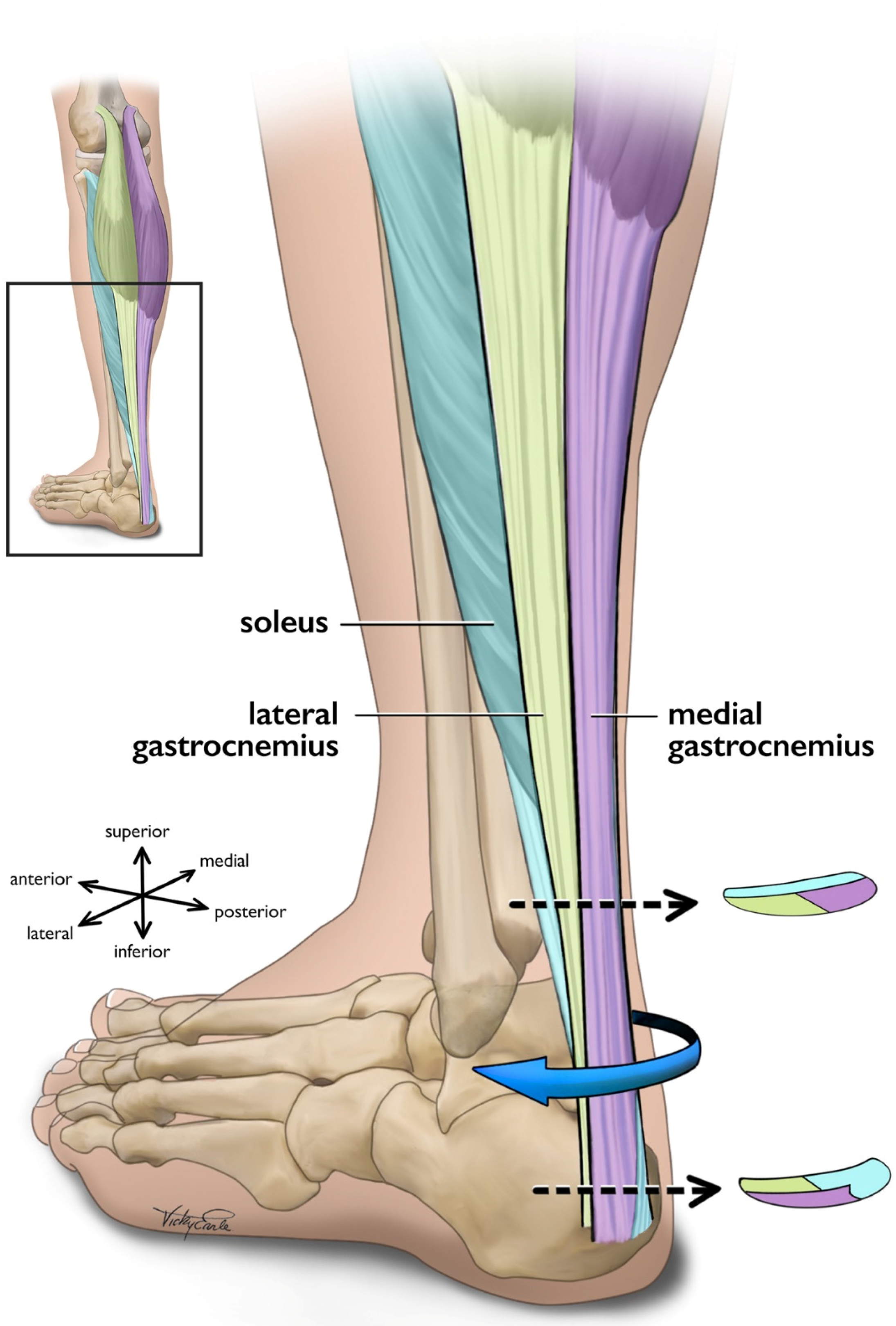
Figure 1. Posterolateral view of the left Achilles tendon and the three subtendons which comprise it. Subtendons rotate in a clockwise fashion traveling distally down the tendon. Cross-sectional views are displayed near the proximal and distal ends of the free tendon, and are based on cadaveric studies [56][82]. The soleus and soleus subtendon are colored teal, the lateral gastrocnemius and associated subtendon chartreuse, and the medial gastroc and its subtendon lavender.
3. Tendon Tissue Remodeling
Despite the complex loading mechanics of the triceps surae MTU, not all loading is detrimental to tendon health. While extrinsic factors contributing to tendon damage appear to be primarily attributable to submaximal cyclic loading, such as those induced by running and other training-related factors [88], targeted tendon loading of adequate magnitude can induce positive changes in tendon morphological, material, and mechanical properties [29][32]. Specifically, mechanotransduction details the body’s ability to translate mechanical loading into structural tissue change via cellular responses [89].
3.1. Healthy Tissue Remodeling
Mechanosensitive cells are responsive to tension, compression, and shear [90]. Loading magnitude [29][32], and perhaps more precisely strain [42][43][44], appears to modulate mechanotransduction in the healthy Achilles tendon. Specifically, strain magnitude, frequency, rate, and duration influence tenocyte biochemical processes [91][92][93] and gene expression [94][95]. For adequately long intervention durations (generally 12 weeks [36]) loads of greater than 70% of maximum voluntary contraction (MVC) [29][32] or strains of 4.5–6.5% [42][43][44] may deliver the appropriate loading-induced tendon stimulus to initiate mechanotransduction pathways; however, the relationship of tendon force and resulting strain can vary substantially between individuals [96][97]. Additionally, strain calculated as the displacement of the gastrocnemius medialis myotendinous junction from its resting length may differ from strain calculated as the change in length of the free tendon, which is more compliant [98][99], and perhaps where the majority of strain occurs. Theoretically, only looking at strain across the free tendon could change the ‘optimal’ adaptation threshold of 4.5–6.5% strain [42][43][44] typically arising from loading programs of greater than 70% of MVC [29][32].
Although the metabolic activity of tendon is low and the structure is typically static, loading-induced stimuli may trigger mechanotransduction and anabolic signaling pathways in the tendon [3]. In particular, the upregulation of insulin-like growth factor (IGF-I), among other growth factors, influences cellular proliferation and matrix remodeling [89][100][101]. Positive matrix remodeling appears to be largely attributable to a net synthesis of type I collagen, thereby making the tendon more load-resistant, though components of the ECM—proteoglycans, glycosaminoglycans, and cross-links—are also influenced by mechanical loading and contribute to macroscopic tendon behaviour through their actions on collagen fibrils [49][100]. Mechanically, longitudinal stiffness (resistance to deformation) increases [29][32][102], and strain for a given tendon force decreases [43][103] in response to increased loading in vivo. Material properties increasing in response to increased loading in vivo include modulus [29][32][102]. Morphologically, tendon CSA increases in response to increased loading in vivo [29][32][102], though limited evidence suggests that transient fluid redistribution may mask this in the short-term [51][104]. Additionally, loading-induced changes may differ along the Achilles tendon as the regional variation in load management [98][105][106] may preferentially activate mechanotransductive pathways leading to region-specific tendon hypertrophy [43][44]. Though still an area of exploration, the opposite could also be the case in that the non-uniform stress distribution within the Achilles tendon could contribute to the location of abnormalities associated with AT [107]. Moreover, while the tendon changes/adaptations described above are primarily related to resistance training, it appears that other types of mechanical loading, such as cyclic loading (e.g., running), can also induce adaptation in the healthy Achilles tendon [108][109]; however, conflicting evidence suggests that some other types of mechanical loading, such as plyometric exercises, may or may not adapt the Achilles tendon in a similar fashion [110][111][112][113][114][115].
3.2. Pathologic Tissue Remodeling
The pathogenesis of tendinopathy appears multifaceted, which has given rise to various pathophysiological theories [36]. Current rhetoric suggests that initial cyclic overloading of the tendon leads to degeneration and disorganization of healthy collagen, which triggers an acute inflammatory response [36][87][101]. If the cyclic overloading is continued without intervention, the tendon pathology worsens through a positive feedback loop of injury to both the original and poor-quality repair tissue, inflammation, and failed repair. Macroscopically, evidence suggests that AT increases tendon CSA [116][117][118] and longitudinal strain [116][117][118], and decreases modulus [116][119], transverse strain [120], longitudinal stiffness [116][118][119], and transverse stiffness [121] in vivo. Taken together, these changes lead to functional deficits across the strength spectrum potentially increasing risk of AT recurrence [122][123][124].
Therapeutic exercise remains one of if not the most effective non-surgical approach for managing AT [1][2]. The suggested mechanism of action is generally considered to be restoration of tendon material, mechanical, and morphological properties similarly to healthy tendon remodeling [36][37][41], thereby improving functional strength [33]. Macroscopically, evidence suggests that targeted mechanical loading decreases tendon thickness [125] and volume [126]; however, there is a paucity of evidence underpinning the restoration of tendinopathic tissue capacity, with most studies focusing on functional and acute analgesic effects [36]. Evidence suggests that abnormal structure (i.e., hypoechoic areas and irregular structure) may normalize in some individuals following a 12-week eccentric exercise protocol [125][127], though the time needed for such changes to occur may vary [38]. Additionally, Cook and colleagues [128] posit that exercise-based adaptation may build capacity in the area of aligned fibrillar structure instead of acting on the area of abnormal structure. Nonetheless, evidence suggests that structural changes do not entirely explain clinical outcomes [129][130]. Building on this idea, O’Neill, Watson, and Barry [37] highlight that tendon structure is not observed to significantly change over the typical intervention period. The researchers further suggest that changes in neuromuscular output may explain clinical benefit, and that training should focus on increasing stiffness of the triceps surae MTU, increasing strength, and shifting the length-tension curve of the triceps surae muscles through sarcomerogenesis. Although still an area of exploration, it appears that therapeutic exercise for AT should focus on improving the mechanical and material properties of the entire MTU thereby simultaneously building strength capacity and neuromuscular control [131].
This entry is adapted from the peer-reviewed paper 10.3390/jcm11164722
References
- De Vos, R.-J.J.; van der Vlist, A.C.; Zwerver, J.; Meuffels, D.E.; Smithuis, F.; Van Ingen, R.; van der Giesen, F.; Visser, E.; Balemans, A.; Pols, M.; et al. Dutch multidisciplinary guideline on Achilles tendinopathy. Br. J. Sports Med. 2021, 55, 1125–1134.
- Martin, R.L.; Chimenti, R.; Cuddeford, T.; Houck, J.; Matheson, J.W.; McDonough, C.M.; Paulseth, S.; Wukich, D.K.; Carcia, C.R. Achilles pain, stiffness, and muscle power deficits: Midportion achilles tendinopathy revision 2018. J. Orthop. Sports Phys. Ther. 2018, 48, A1–A38.
- Ackermann, P.W.; Hart, D.A. Metabolic Influences on Risk for Tendon Disorders; Springer International Publishing: Cham, Switzerland, 2016; ISBN 9783319339436 3319339435.
- Magnusson, S.P.; Narici, M.V.; Maganaris, C.N.; Kjaer, M. Human tendon behaviour and adaptation, in vivo. J. Physiol. 2008, 586, 71–81.
- Alexander, R.M. Energy-saving mechanisms in walking and running. J. Exp. Biol. 1991, 160, 55–69.
- Fukunaga, T.; Kubo, K.; Kawakami, Y.; Fukashiro, S.; Kanehisa, H.; Maganaris, C.N. In vivo behaviour of human muscle tendon during walking. Proc. R. Soc. B Biol. Sci. 2001, 268, 229–233.
- Benjamin, M.; Kaiser, E.; Milz, S. Structure-function relationships in tendons: A review. J. Anat. 2008, 212, 211–228.
- Lacroix, A.S.; Duenwald-Kuehl, S.E.; Lakes, R.S.; Vanderby, R. Relationship between tendon stiffness and failure: A metaanalysis. J. Appl. Physiol. 2013, 115, 43–51.
- Thornton, G.M.; Hart, D.A. The interface of mechanical loading and biological variables as they pertain to the development of tendinosis. J. Musculoskelet. Neuronal Interact. 2011, 11, 94–105.
- Wang, J.H.C. Mechanobiology of tendon. J. Biomech. 2006, 39, 1563–1582.
- Winnicki, K.; Ochała-Kłos, A.; Rutowicz, B.; Pękala, P.A.; Tomaszewski, K.A. Functional anatomy, histology and biomechanics of the human Achilles tendon—A comprehensive review. Ann. Anat. 2020, 229, 151461.
- Willy, R.W.; Halsey, L.; Hayek, A.; Johnson, H.; Willson, J.D. Patellofemoral joint and achilles tendon loads during overground and treadmill running. J. Orthop. Sports Phys. Ther. 2016, 46, 664–672.
- Sinclair, J.; Richards, J.; Shore, H. Effects of minimalist and maximalist footwear on Achilles tendon load in recreational runners. Comp. Exerc. Physiol. 2015, 11, 239–244.
- Almonroeder, T.; Willson, J.D.; Kernozek, T.W. The effect of foot strike pattern on achilles tendon load during running. Ann. Biomed. Eng. 2013, 41, 1758–1766.
- Baxter, J.R.; Corrigan, P.; Hullfish, T.J.; O’Rourke, P.; Silbernagel, K.G. Exercise Progression to Incrementally Load the Achilles Tendon. Med. Sci. Sports Exerc. 2021, 53, 124–130.
- Maffulli, N.; Sharma, P.; Luscombe, K.L. Achilles Tendinopathy: Aetiology and Management. J. R. Soc. Med. 2004, 97, 472–476.
- Selvanetti, A.; Cipolla, M.; Puddu, G. Overuse tendon injuries: Basic science and classification. Oper. Technol. Sport. Med. 1997, 5, 110–117.
- Scott, A.; Squier, K.; Alfredson, H.; Bahr, R.; Cook, J.L.; Coombes, B.; De Vos, R.J.; Fu, S.N.; Grimaldi, A.; Lewis, J.S.; et al. ICON 2019: International Scientific Tendinopathy Symposium Consensus: Clinical Terminology. Br. J. Sports Med. 2020, 54, 260–262.
- De Jonge, S.; Van Den Berg, C.; De Vos, R.J.; Van Der Heide, H.J.L.; Weir, A.; Verhaar, J.A.N.; Bierma-Zeinstra, S.M.A.; Tol, J.L. Incidence of midportion Achilles tendinopathy in the general population. Br. J. Sports Med. 2011, 45, 1026–1028.
- Lopes, A.D.; Hespanhol, L.C.; Yeung, S.S.; Costa, L.O.P. What are the Main Running-Related Musculoskeletal Injuries? Sports Med. 2012, 42, 891–905.
- Nielsen, R.O.; Rønnow, L.; Rasmussen, S.; Lind, M. A prospective study on time to recovery in 254 injured novice runners. PLoS ONE 2014, 9, e99877.
- Lagas, I.F.; Fokkema, T.; Verhaar, J.A.N.; Bierma-Zeinstra, S.M.A.; van Middelkoop, M.; de Vos, R.J. Incidence of Achilles tendinopathy and associated risk factors in recreational runners: A large prospective cohort study. J. Sci. Med. Sport 2020, 23, 448–452.
- Kujala, U.M.; Sarna, S.; Kaprio, J. Cumulative incidence of achilles tendon rupture and tendinopathy in male former elite athletes. Clin. J. Sport Med. 2005, 15, 133–135.
- Kvist, M. Achilles tendon injuries in athletes. Ann. Chir. Gynaecol. 1991, 80, 188–201.
- Caudell, G.M. Insertional Achilles Tendinopathy. Clin. Podiatr. Med. Surg. 2017, 34, 195–205.
- Yin, N.H.; Fromme, P.; McCarthy, I.; Birch, H.L. Individual variation in achilles tendon morphology and geometry changes susceptibility to injury. Elife 2021, 10, e63204.
- Obst, S.J.; Heales, L.J.; Schrader, B.L.; Davis, S.A.; Dodd, K.A.; Holzberger, C.J.; Beavis, L.B.; Barrett, R.S. Are the Mechanical or Material Properties of the Achilles and Patellar Tendons Altered in Tendinopathy? A Systematic Review with Meta-analysis. Sports Med. 2018, 48, 2179–2198.
- Yasui, Y.; Tonogai, I.; Rosenbaum, A.J.; Shimozono, Y.; Kawano, H.; Kennedy, J.G. The Risk of Achilles Tendon Rupture in the Patients with Achilles Tendinopathy: Healthcare Database Analysis in the United States. Biomed. Res. Int. 2017, 2017, 7021862.
- Bohm, S.; Mersmann, F.; Arampatzis, A. Human tendon adaptation in response to mechanical loading: A systematic review and meta-analysis of exercise intervention studies on healthy adults. Sports Med. Open 2015, 1, 7.
- Svensson, R.B.; Heinemeier, K.M.; Couppé, C.; Kjaer, M.; Magnusson, S.P. Effect of aging and exercise on the tendon. J. Appl. Physiol. 2016, 121, 1353–1362.
- McCrum, C.; Leow, P.; Epro, G.; König, M.; Meijer, K.; Karamanidis, K. Alterations in Leg Extensor Muscle-Tendon Unit Biomechanical Properties With Ageing and Mechanical Loading. Front. Physiol. 2018, 9, 150.
- Lazarczuk, S.L.; Maniar, N.; Opar, D.A.; Duhig, S.J.; Shield, A.; Barrett, R.S.; Bourne, M.N. Mechanical, Material and Morphological Adaptations of Healthy Lower Limb Tendons to Mechanical Loading: A Systematic Review and Meta-Analysis. Sport. Med. 2022; in press.
- Malliaras, P.; Barton, C.J.; Reeves, N.D.; Langberg, H. Achilles and patellar tendinopathy loading programmes: A systematic review comparing clinical outcomes and identifying potential mechanisms for effectiveness. Sports Med. 2013, 43, 267–286.
- Habets, B.; van Cingel, R.E.H.; Backx, F.J.G.; van Elten, H.J.; Zuithoff, P.; Huisstede, B.M.A. No Difference in Clinical Effects When Comparing Alfredson Eccentric and Silbernagel Combined Concentric-Eccentric Loading in Achilles Tendinopathy: A Randomized Controlled Trial. Orthop. J. Sports Med. 2021, 9, 23259671211031254.
- Beyer, R.; Kongsgaard, M.; Hougs Kjær, B.; Øhlenschlæger, T.; Kjær, M.; Magnusson, S.P. Heavy slow resistance versus eccentric training as treatment for achilles tendinopathy: A randomized controlled trial. Am. J. Sports Med. 2015, 43, 1704–1711.
- Millar, N.L.; Silbernagel, K.G.; Thorborg, K.; Kirwan, P.D.; Galatz, L.M.; Abrams, G.D.; Murrell, G.A.C.; McInnes, I.B.; Rodeo, S.A. Tendinopathy. Nat. Rev. Dis. Prim. 2021, 7, 1.
- O’Neill, S.; Watson, P.J.; Barry, S. Why are eccentric exercises effective for Achilles tendinopathy? Int. J. Sport. Phys. Ther. 2015, 10, 552–562.
- De Vos, R.J.; Heijboer, M.P.; Weinans, H.; Verhaar, J.A.N.; van Schie, H.T.M. Tendon structure’s lack of relation to clinical outcome after eccentric exercises in chronic midportion Achilles tendinopathy. J. Sport Rehabil. 2012, 21, 34–43.
- Magnussen, R.A.; Dunn, W.R.; Thomson, A.B. Nonoperative treatment of midportion achilles tendinopathy: A systematic review. Clin. J. Sport Med. 2009, 19, 54–64.
- Burton, I.; McCormack, A. The implementation of resistance training principles in exercise interventions for lower limb tendinopathy: A systematic review. Phys. Ther. Sport 2021, 50, 97–113.
- Silbernagel, K.G.; Hanlon, S.; Sprague, A. Current clinical concepts: Conservative management of achilles tendinopathy. J. Athl. Train. 2020, 55, 438–447.
- Arampatzis, A.; Peper, A.; Bierbaum, S.; Albracht, K. Plasticity of human Achilles tendon mechanical and morphological properties in response to cyclic strain. J. Biomech. 2010, 43, 3073–3079.
- Arampatzis, A.; Karamanidis, K.; Albracht, K. Adaptational responses of the human Achilles tendon by modulation of the applied cyclic strain magnitude. J. Exp. Biol. 2007, 210, 2743–2753.
- Bohm, S.; Mersmann, F.; Tettke, M.; Kraft, M.; Arampatzis, A. Human Achilles tendon plasticity in response to cyclic strain: Effect of rate and duration. J. Exp. Biol. 2014, 217, 4010–4017.
- Mousavizadeh, R.; Hojabrpour, P.; Eltit, F.; McDonald, P.C.; Dedhar, S.; McCormack, R.G.; Duronio, V.; Jafarnejad, S.M.; Scott, A. β1 integrin, ILK and mTOR regulate collagen synthesis in mechanically loaded tendon cells. Sci. Rep. 2020, 10, 12644.
- McNeilly, C.M.; Banes, A.J.; Benjamin, M.; Ralphs, J.R. Tendon cells in vivo form a three dimensional network of cell processes linked by gap junctions. J. Anat. 1996, 189, 593–600.
- Wall, M.E.; Weinhold, P.S.; Siu, T.; Brown, T.D.; Banes, A.J. Comparison of cellular strain with applied substrate strain in vitro. J. Biomech. 2007, 40, 173–181.
- Maeda, E.; Ye, S.; Wang, W.; Bader, D.L.; Knight, M.M.; Lee, D.A. Gap junction permeability between tenocytes within tendon fascicles is suppressed by tensile loading. Biomech. Model. Mechanobiol. 2012, 11, 439–447.
- Screen, H.R.C. Hierarchical Approaches to Understanding Tendon Mechanics. J. Biomech. Sci. Eng. 2009, 4, 481–499.
- Pang, X.; Wu, J.P.; Allison, G.T.; Xu, J.; Rubenson, J.; Zheng, M.H.; Lloyd, D.G.; Gardiner, B.; Wang, A.; Kirk, T.B. Three dimensional microstructural network of elastin, collagen, and cells in Achilles tendons. J. Orthop. Res. 2017, 35, 1203–1214.
- Merza, E.; Pearson, S.; Lichtwark, G.; Ollason, M.; Malliaras, P. Immediate and long-term effects of mechanical loading on Achilles tendon volume: A systematic review and meta-analysis. J. Biomech. 2021, 118, 110289.
- Komi, P.V. Relevance of in vivo force measurements to human biomechanics. J. Biomech. 1990, 23, 23–34.
- Komi, P.V.; Fukashiro, S.; Jarvinen, M. Biomechanical loading of Achilles tendon during normal locomotion. Clin. Sports Med. 1992, 11, 521–531.
- Lichtwark, G.A.; Wilson, A.M. Optimal muscle fascicle length and tendon stiffness for maximising gastrocnemius efficiency during human walking and running. J. Theor. Biol. 2008, 252, 662–673.
- Fletcher, J.R.; MacIntosh, B.R. Achilles tendon strain energy in distance running: Consider the muscle energy cost. J. Appl. Physiol. 2015, 118, 193–199.
- Szaro, P.; Witkowski, G.; Śmigielski, R.; Krajewski, P.; Ciszek, B. Fascicles of the adult human Achilles tendon—An anatomical study. Ann. Anat. 2009, 191, 586–593.
- Handsfield, G.G.; Slane, L.C.; Screen, H.R.C. Nomenclature of the tendon hierarchy: An overview of inconsistent terminology and a proposed size-based naming scheme with terminology for multi-muscle tendons. J. Biomech. 2016, 49, 3122–3124.
- Shaw, H.M.; Vázquez, O.T.; McGonagle, D.; Bydder, G.; Santer, R.M.; Benjamin, M. Development of the human Achilles tendon enthesis organ. J. Anat. 2008, 213, 718–724.
- Wren, T.A.L.; Yerby, S.A.; Beaupré, G.S.; Carter, D.R. Mechanical properties of the human achilles tendon. Clin. Biomech. 2001, 16, 245–251.
- Bogaerts, S.; Desmet, H.; Slagmolen, P.; Peers, K. Strain mapping in the Achilles tendon—A systematic review. J. Biomech. 2016, 49, 1411–1419.
- Buehler, M.J. Atomistic and continuum modeling of mechanical properties of collagen: Elasticity, fracture, and self-assembly. J. Mater. Res. 2006, 21, 1947–1961.
- Mosler, E.; Folkhard, W.; Knörzer, E.; Nemetschek-Gansler, H.; Nemetschek, T.; Koch, M.H.J. Stress-induced molecular rearrangement in tendon collagen. J. Mol. Biol. 1985, 182, 589–596.
- Misof, K.; Rapp, G.; Fratzl, P. A new molecular model for collagen elasticity based on synchrotron x- ray scattering evidence. Biophys. J. 1997, 72, 1376–1381.
- Siegel, R.C. Collagen cross linking. Synthesis of collagen cross links in vitro with highly purified lysyl oxidase. J. Biol. Chem. 1976, 251, 5786–5792.
- Reiser, K.; McCormick, R.J.; Rucker, R.B. Enzymatic and nonenzymatic cross-linking of collagen and elastin. FASEB J. 1992, 6, 2439–2449.
- Bailey, A.J.; Paul, R.G.; Knott, L. Mechanisms of maturation and ageing of collagen. Mech. Ageing Dev. 1998, 106, 1–56.
- Holmes, D.F.; Lu, Y.; Starborg, T.; Kadler, K.E. Collagen Fibril Assembly and Function. In Current Topics in Developmental Biology; Academic Press: Cambridge, MA, USA, 2018; Volume 130, pp. 107–142.
- Buehler, M.J. Nanomechanics of collagen fibrils under varying cross-link densities: Atomistic and continuum studies. J. Mech. Behav. Biomed. Mater. 2008, 1, 59–67.
- Herchenhan, A.; Kalson, N.S.; Holmes, D.F.; Hill, P.; Kadler, K.E.; Margetts, L. Tenocyte contraction induces crimp formation in tendon-like tissue. Biomech. Model. Mechanobiol. 2012, 11, 449–459.
- Screen, H.R.C.; Lee, D.A.; Bader, D.L.; Shelton, J.C. An investigation into the effects of the hierarchical structure of tendon fascicles on micromechanical properties. Proc. Inst. Mech. Eng. Part H J. Eng. Med. 2004, 218, 109–119.
- Haraldsson, B.T.; Aagaard, P.; Qvortrup, K.; Bojsen-Moller, J.; Krogsgaard, M.; Koskinen, S.; Kjaer, M.; Magnusson, S.P. Lateral force transmission between human tendon fascicles. Matrix Biol. 2008, 27, 86–95.
- Shim, V.B.; Handsfield, G.G.; Fernandez, J.W.; Lloyd, D.G.; Besier, T.F. Combining in silico and in vitro experiments to characterize the role of fascicle twist in the Achilles tendon. Sci. Rep. 2018, 8, 13856.
- Landin, D.; Thompson, M.; Reid, M. Actions of Two Bi-Articular Muscles of the Lower Extremity: A Review Article. J. Clin. Med. Res. 2016, 8, 489–494.
- Hamner, S.R.; Delp, S.L. Muscle contributions to fore-aft and vertical body mass center accelerations over a range of running speeds. J. Biomech. 2013, 46, 780–787.
- Bohm, S.; Mersmann, F.; Santuz, A.; Arampatzis, A. Enthalpy efficiency of the soleus muscle contributes to improvements in running economy. Proc. R. Soc. B 2021, 288, 20202784.
- Alway, S.E.; MacDougall, J.D.; Sale, D.G.; Sutton, J.R.; McComas, A.J. Functional and structural adaptations in skeletal muscle of trained athletes. J. Appl. Physiol. 1988, 64, 1114–1120.
- Friederich, J.A.; Brand, R.A. Muscle fiber architecture in the human lower limb. J. Biomech. 1990, 23, 91–95.
- Kawakami, Y.; Ichinose, Y.; Fukunaga, T. Architectural and functional features of human triceps surae muscles during contraction. J. Appl. Physiol. 1998, 85, 398–404.
- Albracht, K.; Arampatzis, A.; Baltzopoulos, V. Assessment of muscle volume and physiological cross-sectional area of the human triceps surae muscle in vivo. J. Biomech. 2008, 41, 2211–2218.
- Brand, R.A.; Pedersen, D.R.; Friederich, J.A. The sensitivity of muscle force predictions to changes in physiologic cross-sectional area. J. Biomech. 1986, 19, 589–596.
- Haxton, H.A. Absolute muscle force in the ankle flexors of man. J. Physiol. 1944, 103, 267–273.
- Edama, M.; Kubo, M.; Onishi, H.; Takabayashi, T.; Inai, T.; Yokoyama, E.; Hiroshi, W.; Satoshi, N.; Kageyama, I. The twisted structure of the human Achilles tendon. Scand. J. Med. Sci. Sports 2015, 25, e497–e503.
- Van Gils, C.C.; Steed, R.H.; Page, J.C. Torsion of the human Achilles tendon. J. Foot Ankle Surg. 1996, 35, 41–48.
- Sugisaki, N.; Kawakami, Y.; Kanehisa, H.; Fukunaga, T. Effect of muscle contraction levels on the force-length relationship of the human Achilles tendon during lengthening of the triceps surae muscle-tendon unit. J. Biomech. 2011, 44, 2168–2171.
- Clark, W.H.; Franz, J.R. Do triceps surae muscle dynamics govern non-uniform Achilles tendon deformations? PeerJ 2018, 2018, 5182.
- Bojsen-Møller, J.; Hansen, P.; Aagaard, P.; Svantesson, U.; Kjaer, M.; Magnusson, S.P. Differential displacement of the human soleus and medial gastrocnemius aponeuroses during isometric plantar flexor contractions in vivo. J. Appl. Physiol. 2004, 97, 1908–1914.
- Scott, A.; Backman, L.J.; Speed, C. Tendinopathy: Update on pathophysiology. J. Orthop. Sports Phys. Ther. 2015, 45, 833–841.
- Järvinen, T.A.H.; Kannus, P.; Maffulli, N.; Khan, K.M. Achilles tendon disorders: Etiology and epidemiology. Foot Ankle Clin. 2005, 10, 255–266.
- Khan, K.M.; Scott, A. Mechanotherapy: How physical therapists’ prescription of exercise promotes tissue repair. Br. J. Sports Med. 2009, 43, 247–252.
- Dunn, S.L.; Olmedo, M.L. Mechanotransduction: Relevance to physical therapist practice—Understanding our ability to affect genetic expression through mechanical forces. Phys. Ther. 2016, 96, 712–721.
- Arnoczky, S.P.; Lavagnino, M.; Whallon, J.H.; Hoonjan, A. In situ cell nucleus deformation in tendons under tensile load; a morphological analysis using confocal laser microscopy. J. Orthop. Res. 2002, 20, 29–35.
- Lavagnino, M.; Arnoczky, S.P.; Tian, T.; Vaupel, Z. Effect of Amplitude and Frequency of Cyclic Tensile Strain on the Inhibition of MMP-1 mRNA Expression in Tendon Cells: An In Vitro Study. Connect. Tissue Res. 2003, 44, 181–187.
- Skutek, M.; Van Griensven, M.; Zeichen, J.; Brauer, N.; Bosch, U. Cyclic mechanical stretching modulates secretion pattern of growth factors in human tendon fibroblasts. Eur. J. Appl. Physiol. 2001, 86, 48–52.
- Jones, E.R.; Jones, G.C.; Legerlotz, K.; Riley, G.P. Cyclical strain modulates metalloprotease and matrix gene expression in human tenocytes via activation of TGFβ. Biochim. Biophys. Acta Mol. Cell Res. 2013, 1833, 2596–2607.
- Maeda, E.; Shelton, J.C.; Bader, D.L.; Lee, D.A. Differential regulation of gene expression in isolated tendon fascicles exposed to cyclic tensile strain in vitro. J. Appl. Physiol. 2009, 106, 506–512.
- Arampatzis, A.; Mersmann, F.; Bohm, S. Individualized Muscle-Tendon Assessment and Training. Front. Physiol. 2020, 11, 723.
- Pizzolato, C.; Lloyd, D.G.; Zheng, M.H.; Besier, T.F.; Shim, V.B.; Obst, S.J.; Newsham-West, R.; Saxby, D.J.; Barrett, R.S. Finding the sweet spot via personalised Achilles tendon training: The future is within reach. Br. J. Sports Med. 2019, 53, 11–12.
- Magnusson, S.P.; Hansen, P.; Aagaard, P.; Brønd, J.; Dyhre-Poulsen, P.; Bojsen-Moller, J.; Kjaer, M. Differential strain patterns of the human gastrocnemius aponeurosis and free tendon, in vivo. Acta Physiol. Scand. 2003, 177, 185–195.
- Bojsen-Møller, J.; Peter Magnusson, S. Mechanical properties, physiological behavior, and function of aponeurosis and tendon. J. Appl. Physiol. 2019, 126, 1800–1807.
- Kjaer, M. Role of Extracellular Matrix in Adaptation of Tendon and Skeletal Muscle to Mechanical Loading. Physiol. Rev. 2004, 84, 649–698.
- Magnusson, S.P.; Kjaer, M. The impact of loading, unloading, ageing and injury on the human tendon. J. Physiol. 2019, 597, 1283–1298.
- Wiesinger, H.P.; Kösters, A.; Müller, E.; Seynnes, O.R. Effects of Increased Loading on in Vivo Tendon Properties: A Systematic Review. Med. Sci. Sports Exerc. 2015, 47, 1885–1895.
- Waugh, C.M.; Alktebi, T.; de Sa, A.; Scott, A. Impact of rest duration on Achilles tendon structure and function following isometric training. Scand. J. Med. Sci. Sports 2018, 28, 436–445.
- Fouré, A.; Nordez, A.; Cornu, C. Effects of eccentric training on mechanical properties of the plantar flexor muscle-tendon complex. J. Appl. Physiol. 2013, 114, 523–537.
- Obst, S.J.; Newsham-West, R.; Barrett, R.S. Changes in Achilles tendon mechanical properties following eccentric heel drop exercise are specific to the free tendon. Scand. J. Med. Sci. Sports 2016, 26, 421–431.
- Handsfield, G.G.; Greiner, J.; Madl, J.; Rog-Zielinska, E.A.; Hollville, E.; Vanwanseele, B.; Shim, V. Achilles Subtendon Structure and Behavior as Evidenced From Tendon Imaging and Computational Modeling. Front. Sports Act. Living 2020, 2, 70.
- Gibbon, W.W.; Cooper, J.R.; Radcliffe, G.S. Distribution of sonographically detected tendon abnormalities in patients with a clinical diagnosis of chronic Achilles tendinosis. J. Clin. Ultrasound 2000, 28, 61–66.
- Wiesinger, H.P.; Rieder, F.; Kösters, A.; Müller, E.; Seynnes, O.R. Are Sport-Specific Profiles of Tendon Stiffness and Cross-Sectional Area Determined by Structural or Functional Integrity? PLoS ONE 2016, 11, e0158441.
- Wiesinger, H.P.; Rieder, F.; Kösters, A.; Müller, E.; Seynnes, O.R. Sport-specific capacity to use elastic energy in the patellar and Achilles tendons of elite athletes. Front. Physiol. 2017, 8, 132.
- Kubo, K.; Morimoto, M.; Komuro, T.; Yata, H.; Tsunoda, N.; Kanehisa, H.; Fukunaga, T. Effects of plyometric and weight training on muscle-tendon complex and jump performance. Med. Sci. Sports Exerc. 2007, 39, 1801–1810.
- Fouré, A.; Nordez, A.; Cornu, C. Plyometric training effects on Achilles tendon stiffness and dissipative properties. J. Appl. Physiol. 2010, 109, 849–854.
- Burgess, K.E.; Connick, M.J.; Graham-Smith, P.; Pearson, S.J. Plyometric vs. Isometric Training Influences on Tendon Properties and Muscle Output. J. Strength Cond. Res. 2007, 21, 986–989.
- Wu, Y.K.; Lien, Y.H.; Lin, K.H.; Shih, T.T.F.; Wang, T.G.; Wang, H.K. Relationships between three potentiation effects of plyometric training and performance. Scand. J. Med. Sci. Sports 2010, 20, e80–e86.
- Fouré, A.; Nordez, A.; McNair, P.; Cornu, C. Effects of plyometric training on both active and passive parts of the plantarflexors series elastic component stiffness of muscle-tendon complex. Eur. J. Appl. Physiol. 2011, 111, 539–548.
- Houghton, L.A.; Dawson, B.T.; Rubenson, J. Effects of plyometric training on Achilles tendon properties and shuttle running during a simulated cricket batting innings. J. Strength Cond. Res. 2013, 27, 1036–1046.
- Arya, S.; Kulig, K. Tendinopathy alters mechanical and material properties of the Achilles tendon. J. Appl. Physiol. 2010, 108, 670–675.
- Child, S.; Bryant, A.L.; Clark, R.A.; Crossley, K.M. Mechanical properties of the achilles tendon aponeurosis are altered in athletes with achilles tendinopathy. Am. J. Sports Med. 2010, 38, 1885–1893.
- Chimenti, R.L.; Flemister, A.S.; Tome, J.; McMahon, J.M.; Flannery, M.A.; Xue, Y.; Houck, J.R. Altered tendon characteristics and mechanical properties associated with insertional achilles tendinopathy. J. Orthop. Sports Phys. Ther. 2014, 44, 680–689.
- Kulig, K.; Chang, Y.J.; Winiarski, S.; Bashford, G.R. Ultrasound-Based Tendon Micromorphology Predicts Mechanical Characteristics of Degenerated Tendons. Ultrasound Med. Biol. 2016, 42, 664–673.
- Grigg, N.L.; Wearing, S.C.; Smeathers, J.E. Achilles tendinopathy has an aberrant strain response to eccentric exercise. Med. Sci. Sports Exerc. 2012, 44, 12–17.
- Finnamore, E.; Waugh, C.; Solomons, L.; Ryan, M.; West, C.; Scott, A. Transverse tendon stiffness is reduced in people with Achilles tendinopathy: A cross-sectional study. PLoS ONE 2019, 14, e0211863.
- McAuliffe, S.; Tabuena, A.; McCreesh, K.; O’Keeffe, M.; Hurley, J.; Comyns, T.; Purtill, H.; O’Neill, S.; O’Sullivan, K. Altered strength profile in Achilles tendinopathy: A systematic review and meta-analysis. J. Athl. Train. 2019, 54, 889–900.
- O’Neill, S.; Barry, S.; Watson, P. Plantarflexor strength and endurance deficits associated with mid-portion Achilles tendinopathy: The role of soleus. Phys. Ther. Sport 2019, 37, 69–76.
- Hasani, F.; Vallance, P.; Haines, T.; Munteanu, S.E.; Malliaras, P. Are Plantarflexor Muscle Impairments Present among Individuals with Achilles Tendinopathy and Do They Change with Exercise? A Systematic Review with Meta-analysis. Sports Med.-Open 2021, 7, 18.
- Öhberg, L.; Lorentzon, R.; Alfredson, H. Eccentric training in patients with chronic Achilles tendinosis: Normalised tendon structure and decreased thickness at follow up. Br. J. Sports Med. 2004, 38, 8–11.
- Shalabi, A.; Kristoffersen-Wilberg, M.; Svensson, L.; Aspelin, P.; Movin, T. Eccentric training of the gastrocnemius-soleus complex ion chronic achilles tendinopathy results in decreased tendon volume and intratendinous signal as evaluated by MRI. Am. J. Sports Med. 2004, 32, 1286–1296.
- Öhberg, L.; Alfredson, H. Effects on neovascularisation behind the good results with eccentric training in chronic mid-portion Achilles tendinosis? Knee Surg. Sports Traumatol. Arthrosc. 2004, 12, 465–470.
- Cook, J.L.; Rio, E.; Purdam, C.R.; Docking, S.I. Revisiting the continuum model of tendon pathology: What is its merit in clinical practice and research? Br. J. Sports Med. 2016, 50, 1187–1191.
- Drew, B.T.; Smith, T.O.; Littlewood, C.; Sturrock, B. Do structural changes (eg, collagen/matrix) explain the response to therapeutic exercises in tendinopathy: A systematic review. Br. J. Sports Med. 2014, 48, 966–972.
- Ryan, M.; Bisset, L.; Newsham-West, R. Should we care about tendon structure? The disconnect between structure and symptoms in tendinopathy. J. Orthop. Sports Phys. Ther. 2015, 45, 823–825.
- Murphy, M.; Travers, M.; Gibson, W.; Chivers, P.; Debenham, J.; Docking, S.; Rio, E. Rate of Improvement of Pain and Function in Mid-Portion Achilles Tendinopathy with Loading Protocols: A Systematic Review and Longitudinal Meta-Analysis. Sports Med. 2018, 48, 1875–1891.
This entry is offline, you can click here to edit this entry!
