Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Waste is a heterogeneous and complex matrix, the selective isolation of, for example, polyphenolic compounds, is challenging due to its energy efficiency and at least partially its selectivity. Extraction is handled as an emerging technology in biorefinery approaches. Conventional solid liquid extraction with organic solvents is hazardous and environmentally unfriendly. New extraction methods and green solvents open a wider scope of applications.
- waste
- residue
- bioactive molecules
- extraction
1. Introduction
To overcome the growing gap between environmental sustainability and economic growth and to achieve the transition from the current linear economy to a circular economy, the development of a circular bio-based industry, where biomass and its byproducts can be processed into viable bioenergy, biochemicals, biomaterials, feed, and food, is mandatory. This includes the development of specially designed products and complete recyclability. In the best case, these products are already manufactured from recycled sustainable resources [1][2]. At present, an average of about 8.6% of the global material input, which is about 8.4 Gt, is recycled [3]. In 2019 and 2020, about 9.7% and 9.1%, respectively, were kept in the cycle. To keep global warming under 2 °C and to reach the goal of sustainability in 2050, the circularity gap has to be closed by 2030 by an additional 8.6% recycling rate [4]. Besides the development of new technologies and pathways, the redesign of traditional linear economies for better recyclability and further substitution of fossil-based block chemicals with sustainable counterparts is an approach to start with. Every step further will affect the material balance, leading to the desired recycle ratio of 1. Figure 1 shows a schematic representation of a complete transformation to a circular economy.
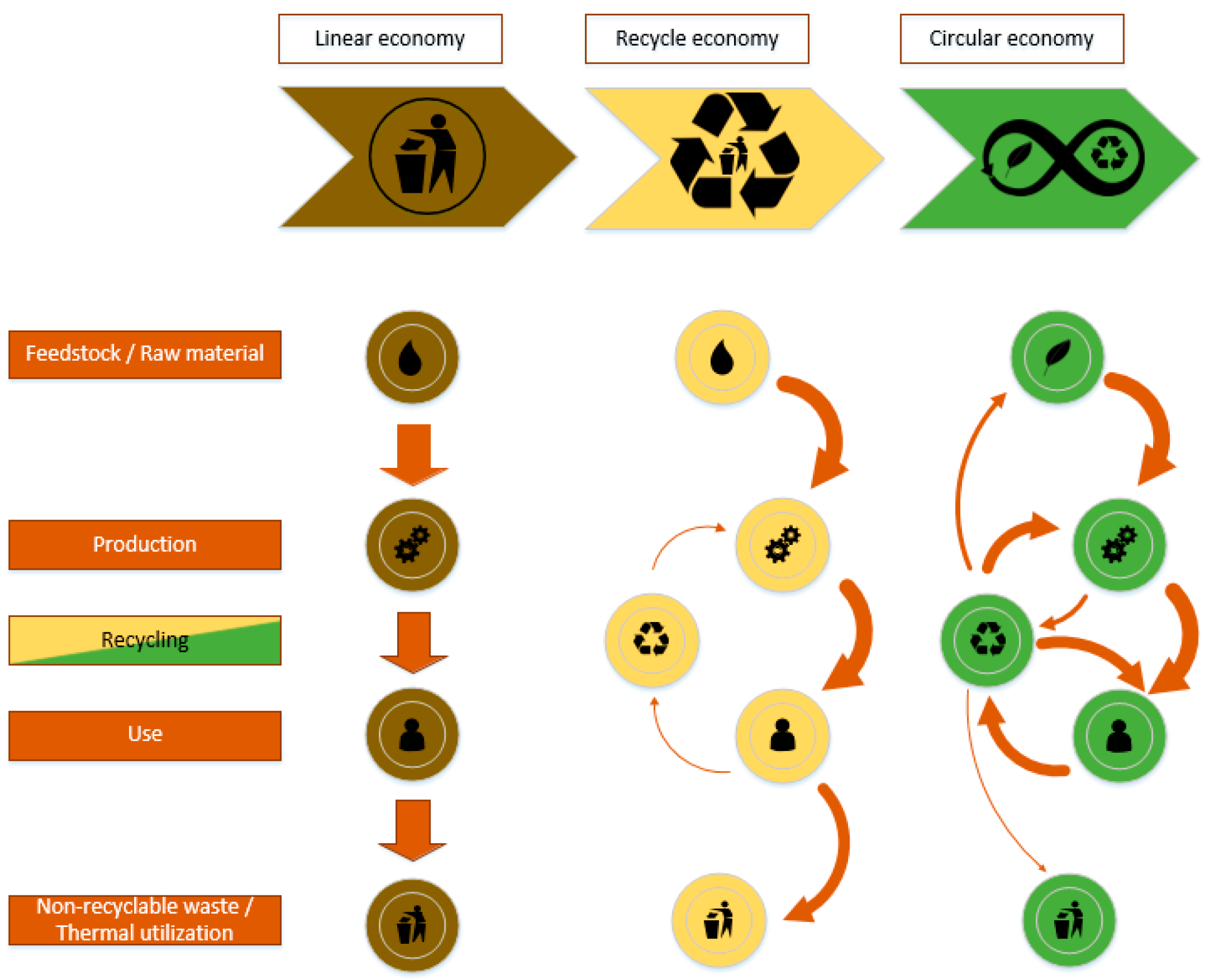
Figure 1. Linear, recycle and circular economy concept.
One tool within this transformation from a linear to a circular, and from a fossil based into a bio-based economy, is biorefineries. Here, renewable feedstock is processed to produce energy and marketable products [5]. In general, all streams generated during downstream processing are converted to marketable intermediates or products. Further, green engineering and green chemistry principles need to be integrated into the overall concept [6]. The most promising feedstocks for biorefineries are waste streams and residues from agricultural production. Approximately 30–40% of the primary agricultural biomass is residues after/during production. Due to their high uniformity, wastes from the bio-based industries are of special interest. An important factor for a positive evaluation is that these feedstocks do not accelerate deforestation or lead to a conflict in land use with food production [7], but the competition with relatively cheap fossil raw materials and the unique and complex chemical structure of biomass requires more effort to receive certain products [8]. In the first approach, biorefineries use agricultural products, mainly edible biomass resources, for the conversion into energy and materials. The competition with food supply, rising basic food prices, as well as deforestation of woodland for agricultural supply and its devastation of formerly fertile land with mono-cultural plantations, paid its tribute in social acceptance of the products all over the world [5][9][10]. Biorefineries based on second and third biomass generations, which do not compete with land use or food production, are now state of the art. The raw materials consist of residues from different agricultural, municipal, and industrial sources as well as algae [10][11]. To help stakeholders and industries classify biorefineries and to develop business opportunities, the IEA Biorefinery Task 42 developed a classification guideline that summarizes a wide range of feedstocks, processes, intermediates, and potential products for biorefinery valorization pathways. The utilization of renewable resources depends on highly interconnected pillars, namely environmental, social, and economic factors.
2. Isolation of Bioactive Molecules from Waste Streams
The isolation of bioactive molecules from waste streams is complex. The main topics that need to be considered when working with industrial and agricultural waste streams are:
- Unsteady chemical composition of biomass across the seasons;
- Varying supply masses, and;
- Low concentration of targeted compounds.
To tackle these topics, a combination of different unit operations for pretreatment and isolation is needed. Here, mechanical, mass transfer, chemical, and biochemical unit operations are used. Which unit operation is used for the isolation of a single or a group of bioactive molecules highly depends on the physical and the chemical properties thereof [12].
2.1. Pre-Treatment
To be able to extract (bioactive) molecules from waste streams an effective pretreatment is needed. Methods that are used for this purpose are electric-assisted or biologic-assisted pretreatments like high voltage electric discharge (HVED), hydrolysis, or fermentation.
2.1.1. Electric Discharge Extraction
HVED and other electrical-assisted extraction methods, such as pulsed electric discharge extraction (PEF) or ohmic heating extraction (OHM), damage the cell wall and fragment the cell. It is an effective pretreatment of feedstocks for the valorization of wet plant tissues [13]. Depending on the parameters, energy input, pH-value, gap distance of the electrodes, and electrical field intensity, HVED is also able to degrade the targeted compounds. A range of 2–3 mm and a voltage of 13 kV showed the best results for the performed extraction [14][15]. Pataro et al. investigated the pretreatment of wet tomato tissue with PEF followed by an extraction with acetone and ethyl lactate. Ethyl lactate turned out to be a good solvent after moderate intensity of 5 kV/cm and 5 kJ/kg for the recovery of carotenoids [13]. When Zhang et al. compared HVED, PEF, and UAE, the main outcome was that UAE led to the highest extraction efficiency [16].
The solvent composition is the most important factor for the pretreatment with electric discharges, as the solvent has to be conductive. Therefore, water is the most suitable solvent for PEF pretreatment [17]. For the verification of the PEF pretreatment, experiments with control groups without PEF were conducted, and PEF showed a 55.8% higher extraction efficiency for flavan–3–ols, a 64.34% higher content of flavonoids, a 68.39% higher concentration of phenolic acids, and a 61.20% higher content of total free phenolics. A coupled pretreatment, including MAE or UAE with PEF, is suggested to achieve higher yields [18].
2.1.2. Hydrolysis & Fermentation
The majority of lignocellulosic residues are currently used as an energy source to produce heat and power by burning or are converted into bioethanol or biogas [19].
When dealing with lignocellulosic feedstocks, lignin needs to be removed before the cellulose and hemicellulose can be degraded to sugars that can be further utilized. Lignin removal can either be performed chemically or biochemically. The research focus lies on chemical conversion routes, but biochemical degradation is less harmful to other substances present in lignocellulosic waste streams than chemical treatment [20]. The degradation of lignin, cellulose, and hemicellulose by enzymes and bacteria leads to the formation of low molecular weight components and bioactive molecules either by the degradation process itself, or as secondary metabolites of the enzyme or bacteria activity [21].
The major unit operations for the valorization of protein-rich lignocellulosic materials are hydrolysis, fermentation, and anaerobic digestion. The lignocellulosic materials are hence the basis for biofuel, especially for bioethanol production [22].
For the release of bioactive compounds, which are embedded to cell wall matrices or to cell cytoplasm with polymers, enzymatic hydrolysis can be an effective pretreatment step. Hydrolysis is a part of enzyme-assisted extraction [23]. Enzymatic hydrolysis is an especially important tool for bioactive peptides because the bioactive parts of the protein have to be removed from the parental part without losing its bioactivity [24]. Other polyphenolic compounds, e.g., procyannidin, can be released into aqueous solutions after the depolymerization processes of pectin [23]. The hydrolysis of animal and plant material is therefore crucial for the release of some bioactive compounds which would be inactive and inaccessible in their natural form.
Although fermentation mainly focuses on the degradation of lignocellulosic compounds, there are ambitions to use it as a pretreatment step for the valorization of bioactive molecules. Besides the before mentioned EAE, solid state fermentation (SSF) is the second investigated natural-driven pretreatment step prior to extraction. Results show that the extraction efficiency increase is highly affected by the strain, feedstock, and used extraction technology. While Gassara et al. reported an improvement in aqueous solid–liquid extraction [25], Kitryte et al. [26] and Fernandes et al. [20] observed a decrease in the extraction efficiency of polyphenolic compounds when the feed was hydrolyzed or fermented prior to extraction. Sepulveda et al. showed that the concentration of flavonoids and phenolic compounds increased during the first 12–54 h. Afterwards, the concentrations decreased, a reason therefore could be the demand of the microorganisms. After the available sugars were fermented, the microorganisms started to degrade other available compounds, such as phenolic compounds [27]. To be able to ferment the biomass, it is important to eliminate or significantly reduce inhibiting components. Citrus peels for, example, were first hydro-distillated to ensure that bacteria-inhibiting properties of essential oils were removed. The essential oil was then collected as a valuable byproduct [28]. El Kantar et al. investigated the simultaneous extraction of polyphenolic compounds and fermented sugars by using HVED and EAE. They concluded that the simultaneous use of both technologies hinders the efficiency of the extraction process. A HVED treatment turned out to be beneficial for further EAE extraction processing [14].
2.2. Extraction
Solid/liquid extraction is the unit operation of choice when bioactive molecules are to be isolated from a solid waste stream. Certain pretreatment steps, such as washing, drying, and milling, are needed to ensure a high surface area and good penetration of the liquid phase. As part of the sustainable development goals, the EU green deal and its national counterparts, industry, and academic institutions are encouraged research on and to substitute conventional, toxic environmental hazardous solvents with sustainable, harmless solvents, such as water, ethanol, ethyl lactate, and the like. Solvents such as water, ethanol, ethyl lactate, or supercritical CO2 can be used for the isolation of bioactive molecules. Due to these solvents, further utilization of the isolated molecules in the food or pharma industry can be done without any limitations. Conventional solvents such as hexane, isopropanol, or methanol lead to limitations in the utilization of the extracted products [29]. Therefore, postprocessing of compounds extracted with conventional solvents is needed. Besides the well-known solid–liquid extraction techniques (SLE) maceration, soxhlet-extraction (SE), hydro-(HD) and steam-distillation (SD), in the last decades’ new SLE techniques, such as systems using new (green) solvents, e.g., deep eutectic solvent (NADES) or aqueous two-phase extraction (ATPE), ultrasound-assisted (UAE), microwave-assisted (MAE), agitated bed extraction (ABE), pressurized sub- (PLE), and supercritical extraction (SFE) with water, ethanol, or CO2, steam explosion extraction (SE), EAE, pulsed electrical discharge extraction (PEF) and HVED, including ohmic heating, have been developed. All these techniques offer beneficial phase transport and higher contact areas and, therefore, increase the yield, lower the extraction time, and decrease the solvent consumption. The main parameters for the extraction are, besides the solvent itself, the extraction time, the pretreatment, the pH-value, the solid/liquid ratio, and the temperature [30].
SE and SLE are often used as reference processes to evaluate new processes or solvents, such as UAE and MAE, or by using acidified liquids, deep eutectic solvents, and oils. A lot of research is compressed into solid liquid, microwave, and ultrasound-assisted extraction methods. For next generational production plants, it has become important to exchange their fossil-based processes and solvents for renewable ones. MAE, UAE, and electric-assisted extraction ensure an increased permeability of the solvent by disrupting the cells [18][31].
Besides the extraction process itself, solvents are crucial for the selection and the environmental footprint of an extraction process. The development and utilization of green solvents in extraction is under investigation. The water solubility and the recovery or back extraction step are two main points.
The substitution of hexane is of importance for different industries. Ozturk et al., for example, investigated the limonene extraction from orange peel. Besides supercritical CO2, cyclopentyl–methyl–ether was mentioned as a green alternative [32]. Methanol and ethanol are powerful solvents for the extraction of polar polyphenolic compounds [18][26][33][34][35][36]. Zivkovic et al. reported a beneficial extraction behavior with water and an ethanol concentration of up to 40% for polyphenolic compounds. Higher alcohol concentrations led to decreasing extraction efficiencies [37]. The use of sunflower and olive oil was shown to be highly effective in combination with UAE to extract lycopene, also in comparison with conventional organic solvents [38][39][40][41]. The results showed that, besides the extraction time, the ultrasonic intensity had the highest influence [40][42]. Aqueous two-phase extraction is based two phases that mainly consist of water. The phase composition mainly uses alcohols + salts or ionic liquids + salts [30][43][44]. In pure aqueous systems, ultrasound enhances the extraction for sugars but not for phenolic compounds. In systems with ethanol, the extraction yield was significantly higher for both substance groups, while in both systems with conventional SLE, the extraction yield remained the same. In further investigation and comparison with enhanced ATPE systems, phenolic compounds were enriched in the ethanol-rich top phase, while sugars were enriched in the salt-rich bottom phase. Additionally, lignans were selectively extracted to the top phase, while carbohydrates and proteins remained in the bottom phase [43][44]. The extraction capacity of the ethanol-rich phase can be adjusted by the amount of salt in the second phase. A high amount of salt led to a worse affinity of polyphenols for the ethanol rich phase. Up to 4 wt% ionic liquids were added to the salt-rich phase, which led to an increase of the extraction efficiency from 59.65 to 97.12% [30]. Natural deep eutectic solvents are handled as a green alternative for a lot of compounds. The extraction efficiency, however, depends on the viscosity and, hence, on the water content, which can be adjusted by changing either the molar ration of the NADES or the substances forming the NADES [36][45][46][47][48][49]. It is reported that the water content undergoes a maximum with respect to the extraction efficiency for the extraction of caffeoylmalic acid, psoralic acid–glucoside, rutin, psoralen, and bergapten with glycerol:xylitol:D–(–)–fructose (3:3:3 mol ratio). A maximum of 20% was reported. In conclusion, this maximum is referred to the lower interaction of the NADES with the target compounds [36][46][49]. The water content and the polarity are connected, while cholinium-based NADES offer a low polarity, which is related to an optimal water content of 50%, organic acid-based NADES, which have a higher polarity, show an optimal water content of 25% [46]. NADES showed significantly higher solubility of compounds than water and superior extraction efficiency compared to conventionally used methanol [49]. Although there is still a lot of research needed, the studies show that there is a wide range of green solvents that can be applied in solid/liquid extraction. By the solvent selection, the pH-value becomes important [39][46][50][51]. It is well known that the pH-value is a parameter that highly influences extraction behavior, for example, phenolic compounds are more likely to be extracted at pH-values of around 3–4 [43][44].
Besides the solvent and the extraction method, the solid/liquid ratio is the next influencing factor on the extraction yield [37]. The optimum solvent/solid ration depends on the chosen system. While Ran et al. reported a solid/liquid ratio of 1:20 for ATPS as optimal, Xi et al. demonstrated that this was their lower limit using HVED with water. A general trend cannot be formulated. The systems, solvents, and extracted compounds are too different. It can be concluded that the solid/liquid ratio is one of the key factors for the evaluation of extraction systems [15][30][32][44][52][53]. As an example, ethanol showed solid/liquid ratio independent extraction behavior for the total phenolic content from cranberries, whereas the optimal solid/liquid ratio for the extraction with methanol was between 1:90 and 1:120 [54]. This example shows that the solvent as well as the solvent ratio can make a big difference. High solvent to liquid ratios do not automatically result in good extraction behavior. In addition, the environmental impact has to be considered. In many experiments, the ratio of 1:20 to 1:30 was reported to be beneficial. For UAE and MAE, an increased solvent load led to a decreasing extraction efficiency, which is based on the decreasing energy absorption of the solid material with higher solvent loads [52].
Rahimi et al. and Amyrgialaki et al. stated that the extraction time had a significant influence on the yield of polyphenolic compounds [33][39][40]. During the optimization process, new enhanced extraction processes showed that quite a similar yield could be achieved within a much shorter time, meaning that the phase equilibrium is reached faster than compared to conventional solvents in SE [43]. The extraction times are influenced by the pretreatment of the feedstock. Data show that long pretreatment times with sonication or microwaves lead to a decreased phenolic compound release or/and a degradation processes thereof [44][55][56]. The effective area and the diffusion path length depend on the particle size, hence, the optimal sonication time depends on the particle size [48].
The temperature and, in the case of MAE, the power level of the microwaves [33], are important factors for the extraction [31][37][52]. Higher temperatures show higher extraction yield due to increased diffusivity by loosening the cell wall components [57]. A slightly worse performance of MAE compared to UAE is a result of the higher thermal input of MAE, and that above temperatures of 50 to 65 °C degradation of most bioactive molecules occur [30][42][51][57]. By optimizing the temperature profile with continuous adaption of the MAE power level, better MAE performance can be achieved [47]. Extraction, in general, benefits from higher temperatures due to better solubility of phenolic compounds, higher diffusion rates, improved viscosity of the solvent, and decreased surface tension [47], further, the targeted compounds and solvents used distinguish the extraction efficiency [36].
Pressurized subcritical liquid extraction is an efficient extraction method compared to SLE, UAE, and MAE. As with solvents, water is most often used. Extractions with water can reach up to 9 times higher yields compared to MAE and UAE [58]. An even better performance of PLE, up to 13 times, was achieved by adding 30% NADES to water [59]. As an example, phenolic compounds were extracted from kiwi peel and pomace residues and the results showed that the acidity of the solvent has a big influence on the efficiency. In comparison with conventional SLE extraction using ethanol, methanol, or acetone as solvent [60][61], PLE with supercritical water outperformed acetone as the best solvent with a four-times higher extraction efficiency [60].
The main influencing parameters for supercritical CO2 extraction are the addition of a cosolvent and the pressure. With elevated pressure and temperature, the selectivity of the solvent increases due to changed polarity [62][63]. The extraction of phenolic compounds from orange and blackberry pomace was investigated, and the main outcomes of the studies were that with increasing temperature, acidity, and the addition of water or ethanol as a cosolvent, the extraction efficiency increases [62][64][65][66]. Nevertheless, it was discovered that the antioxidative capacity, contrary to the higher phenolic content, decreased with higher pressure. Further, the pretreatment influenced the extraction yield. Compared to dried, the fermented pomace contained simpler molecules with better water solubility and better results for the antioxidative activity. By using more polar cosolvents, a two-times higher yield of total phenolic compounds was achieved [63]. Compared to PLE and SE for black chokeberry, SFE showed a significantly lower efficiency in the extraction of total phenolics. Through the addition of more polar cosolvents, the efficiency of SFE was significantly increased, but was still lower compared to PLE [62][63]. However, SFE is considered the most selective extraction method for monomeric anthocyanins from juçara residues compared to SE, UAE, PLE, and ABE [64]. Further, SFE needed 78% less extraction time and 10 times less EtOH for the extraction of phenolic compounds from orange pomace, which makes it the more sustainable and economical process [63].
2.3. Pectin Extraction
Pectin is a complex mixture of polysaccharides, mainly galacturonic acid, and is present in the cell walls of plant tissues. Its main function is to keep cells together and to help ripening fruits keep their shape [67]. It is industrially used as a thickener, texturizer, emulsifier, stabilizer, and gelling agent in the food industry as well as a pharmaceutical agent to decrease blood cholesterol, heart disease, and gallstones and to soothe pain [68]. Figure 2 gives an overview of the most important/regularly used processes for the isolation of pectin from, e.g., citrus fruit, mango, or banana peel [69][70][71].
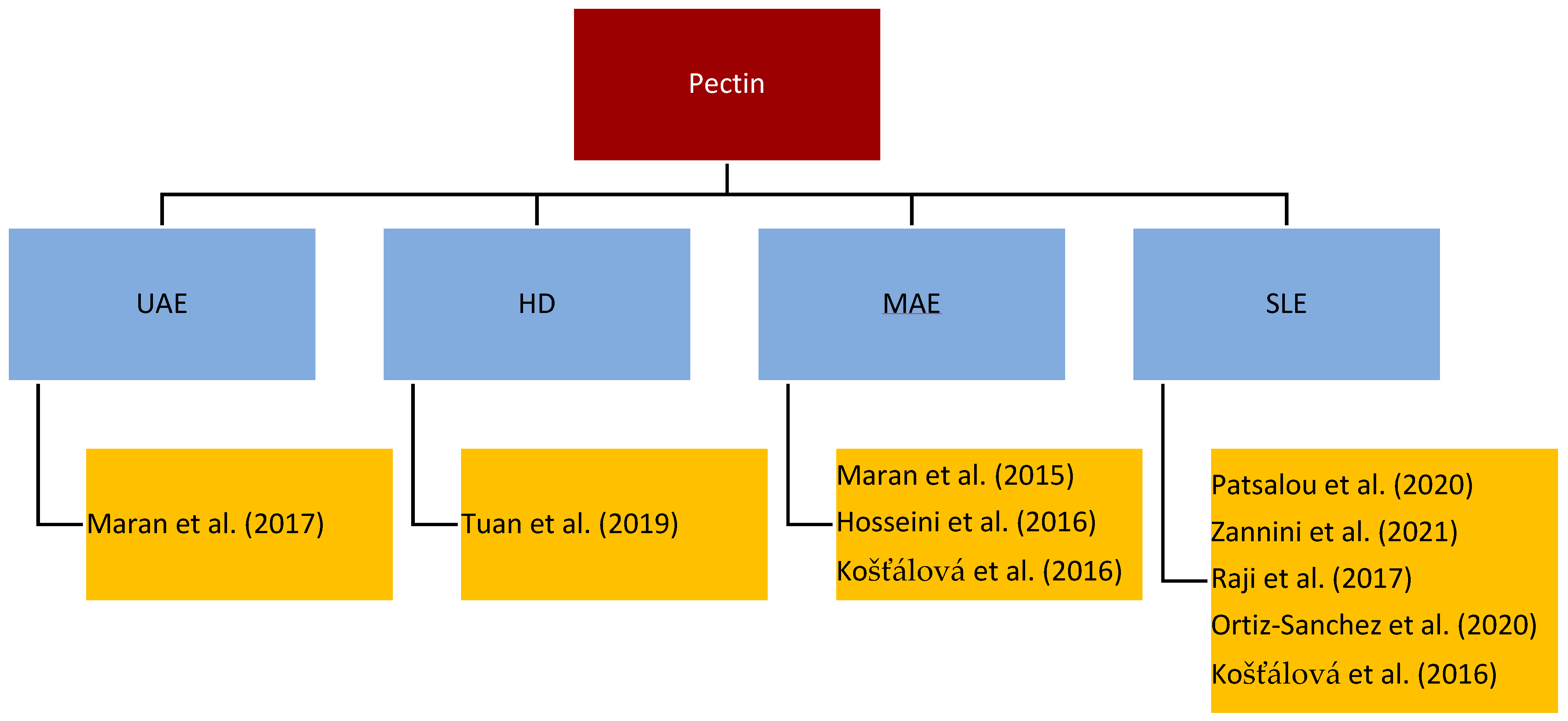
Figure 2. Literature overview of pectin extraction.
The main influencing factor of pectin is the degree of esterification (DE), which tackles the functionality, morphology, and bioactivity of pectin. The power duration of the radiation and the pH-value were identified as important factors for the extraction efficiency, the DE, and the yield of pectin. The higher the radiation power and time, the higher the yield but the lower the DE [71]. Further, a decrease of the extraction time by 83% with a constant yield by using MAE compared to conventional hot acid extraction for medium DE was reported [69]. As for other bioactive molecules, the microwave power has to be limited to control the temperature and to avoid fast degradation of the pectin [70]. Raji et al. identified the type of acid as an important factor and confirmed that the pH-value influences the DE and the extraction yield. For the esterification, citric acid, tartaric acid, acetic acid, lactic acid, nitric acid, phosphoric acid, and sulfuric acid were tested. Citric acid was confirmed to work best for the extraction of pectin [72][73][74]. Many acids lead to pectin depolymerization, like nitric or sulphuric acid. Acetic acid was investigated as an alternative. High acid concentrations lead to high DE. Zannini et al. investigated that a concentration of 3% acetic acid showed a high yield with high DE. Therefore, they concluded that acetic acid is a good alternative to conventionally used organic solvents [73][75]. The yields with citric acid compared to stronger acids, such as HCl, are much lower according to the weaker ability of attacking the cell membrane, but more effective than with nitric acid [74][75]. Weaker organic acids are also more environmentally friendly and cause less corrosion on equipment than their strong inorganic counterparts. Tuan et al. investigated the simultaneous extraction of pectin and essential oils under the influence of citric acid. The citric acid showed no influence on the extracted oil; hence, a simultaneous approach can be implemented [74].
2.4. Pyrolysis
To achieve closed material loops, the solid residues from extraction can still be further utilized. Depending on the used solvents, the residues from extraction can still contain valuable molecules, such as sugars, and not only extracted bioactive molecules. Pyrolysis is used to extract bioactive molecules and to remove agricultural and landscape management wastes, especially waste from greenhouse production, which is mostly not further needed for soil regeneration [76]. In the case of transforming lignocellulosic material to biofuels, pyrolysis is one of the most explored possibilities [77]. The main products from pyrolysis are biochar, condensed biooils, and incondensable gases. Parts of these gases can be further utilized as syngas and industrial energy carriers [76][78]. The focus of today’s biorefineries, and especially the development goals, is on the material utilization of bio-oils. Therefore, single or groups of bioactive compounds have to be isolated or separated, which then can be utilized as pesticides [76][79][80][81][82][83], as antimicrobial agents and preservatives [82][83][84], for medical treatments [81][85], or as a source for precoursers and chemicals [81][86][87].
This entry is adapted from the peer-reviewed paper 10.3390/pr10081668
References
- Kaza, S.; Yao, L.; Bhada-Tata, P.; van Woerden, F.; Lonkova, K.; Morton, J.; Poveda, R.A.; Sarraf, M.; Malkawi, F.; Harinath, A.S.; et al. What a Waste 2.0: A Global Snapshot of Solid Waste Management to 2050; World Bank Group: Washington, DC, USA, 2018; ISBN 978-1-4648-1329-0.
- Attard, T.M.; Clark, J.H.; McElroy, C.R. Recent developments in key biorefinery areas. Curr. Opin. Green Sustain. Chem. 2020, 21, 64–74.
- Haigh, L.; de Wit, M.; Hoogzaad, J.; von Daniels, C.; Colloricchio, A.; Heidtmann, A. Circularity Gap Report 2021. Available online: https://www.circularity-gap.world/2021#downloads (accessed on 21 August 2021).
- De Wit, M.; Hoogzaad, J.; von Daniels, C. Circularity Gap Report 2020. Available online: https://www.circularity-gap.world/2020 (accessed on 21 February 2021).
- De Jong, E.; Stichnothe, H.; Bell, G.; Jørgensen, H. Bio-Based Chemicals: A 2020 Update; IEA: Wageningen, The Netherlands, 2020; ISBN 978-1-910154-69-4.
- Chen, T.-L.; Kim, H.; Pan, S.-Y.; Tseng, P.-C.; Lin, Y.-P.; Chiang, P.-C. Implementation of green chemistry principles in circular economy system towards sustainable development goals: Challenges and perspectives. Sci. Total Environ. 2020, 716, 136998.
- Joelsson, E.; Wallberg, O.; Börjesson, P. Integration potential, resource efficiency and cost of forest-fuel-based biorefineries. Comput. Chem. Eng. 2015, 82, 240–258.
- Budzianowski, W.M. High-value low-volume bioproducts coupled to bioenergies with potential to enhance business development of sustainable biorefineries. Renew. Sustain. Energy Rev. 2017, 70, 793–804.
- Tomei, J.; Helliwell, R. Food versus fuel? Going beyond biofuels. Land Use Policy 2016, 56, 320–326.
- Bharathiraja, B.; Chakravarthy, M.; Ranjith Kumar, R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Praveen Kumar, R.; Palani, S. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653.
- Cuevas-Castillo, G.A.; Navarro-Pineda, F.S.; Baz Rodríguez, S.A.; Sacramento Rivero, J.C. Advances on the processing of microalgal biomass for energy-driven biorefineries. Renew. Sustain. Energy Rev. 2020, 125, 109606.
- Galanakis, C.M. Recovery of high added-value components from food wastes: Conventional, emerging technologies and commercialized applications. Trends Food Sci. Technol. 2012, 26, 68–87.
- Pataro, G.; Carullo, D.; Falcone, M.; Ferrari, G. Recovery of lycopene from industrially derived tomato processing by-products by pulsed electric fields-assisted extraction. Innov. Food Sci. Emerg. Technol. 2020, 63, 102369.
- El Kantar, S.; Boussetta, N.; Rajha, H.N.; Maroun, R.G.; Louka, N.; Vorobiev, E. High voltage electrical discharges combined with enzymatic hydrolysis for extraction of polyphenols and fermentable sugars from orange peels. Food Res. Int. 2018, 107, 755–762.
- Yan, L.-G.; Deng, Y.; Ju, T.; Wu, K.; Xi, J. Continuous high voltage electrical discharge extraction of flavonoids from peanut shells based on “annular gap type” treatment chamber. Food Chem. 2018, 256, 350–357.
- Zhang, R.; Lebovka, N.; Marchal, L.; Vorobiev, E.; Grimi, N. Pulsed electric energy and ultrasonication assisted green solvent extraction of bio-molecules from different microalgal species. Innov. Food Sci. Emerg. Technol. 2020, 62, 102358.
- Redondo, D.; Venturini, M.E.; Luengo, E.; Raso, J.; Arias, E. Pulsed electric fields as a green technology for the extraction of bioactive compounds from thinned peach by-products. Innov. Food Sci. Emerg. Technol. 2018, 45, 335–343.
- Martín-García, B.; Tylewicz, U.; Verardo, V.; Pasini, F.; Gómez-Caravaca, A.M.; Caboni, M.F.; Dalla Rosa, M. Pulsed electric field (PEF) as pre-treatment to improve the phenolic compounds recovery from brewers’ spent grains. Innov. Food Sci. Emerg. Technol. 2020, 64, 102402.
- Bilal, M.; Wang, Z.; Cui, J.; Ferreira, L.F.R.; Bharagava, R.N.; Iqbal, H.M.N. Environmental impact of lignocellulosic wastes and their effective exploitation as smart carriers—A drive towards greener and eco-friendlier biocatalytic systems. Sci. Total Environ. 2020, 722, 137903.
- Fernandes, J.M.C.; Fraga, I.; Sousa, R.M.O.F.; Rodrigues, M.A.M.; Sampaio, A.; Bezerra, R.M.F.; Dias, A.A. Pretreatment of Grape Stalks by Fungi: Effect on Bioactive Compounds, Fiber Composition, Saccharification Kinetics and Monosaccharides Ratio. Int. J. Environ. Res. Public Health 2020, 17, 5900.
- Xu, J.; Dai, L.; Gui, Y.; Yuan, L.; Ma, J.; Zhang, C. Towards a waste-free biorefinery: A cascade valorization of bamboo for efficient fractionation, enzymatic hydrolysis and lithium-sulfur cathode. Ind. Crop. Prod. 2020, 149, 112364.
- Cheng, F.; Brewer, C.E. Conversion of protein-rich lignocellulosic wastes to bio-energy: Review and recommendations for hydrolysis + fermentation and anaerobic digestion. Renew. Sustain. Energy Rev. 2021, 146, 111167.
- Hammed, A.M.; Jaswir, I.; Amid, A.; Alam, Z.; Asiyanbi-H, T.T.; Ramli, N. Enzymatic Hydrolysis of Plants and Algae for Extraction of Bioactive Compounds. Food Rev. Int. 2013, 29, 352–370.
- Cruz-Casas, D.E.; Aguilar, C.N.; Ascacio-Valdés, J.A.; Rodríguez-Herrera, R.; Chávez-González, M.L.; Flores-Gallegos, A.C. Enzymatic hydrolysis and microbial fermentation: The most favorable biotechnological methods for the release of bioactive peptides. Food Chem. 2021, 3, 100047.
- Gassara, F.; Ajila, C.M.; Brar, S.K.; Verma, M.; Tyagi, R.D.; Valero, J.R. Liquid state fermentation of apple pomace sludge for the production of ligninolytic enzymes and liberation of polyphenolic compounds. Process Biochem. 2012, 47, 999–1004.
- Kitrytė, V.; Narkevičiūtė, A.; Tamkutė, L.; Syrpas, M.; Pukalskienė, M.; Venskutonis, P.R. Consecutive high-pressure and enzyme assisted fractionation of blackberry (Rubus fruticosus L.) pomace into functional ingredients: Process optimization and product characterization. Food Chem. 2020, 312, 126072.
- Sepúlveda, L.; Laredo-Alcalá, E.; Buenrostro-Figueroa, J.J.; Ascacio-Valdés, J.A.; Genisheva, Z.; Aguilar, C.; Teixeira, J. Ellagic acid production using polyphenols from orange peel waste by submerged fermentation. Electron. J. Biotechnol. 2020, 43, 1–7.
- Patsalou, M.; Chrysargyris, A.; Tzortzakis, N.; Koutinas, M. A biorefinery for conversion of citrus peel waste into essential oils, pectin, fertilizer and succinic acid via different fermentation strategies. Waste Manag. 2020, 113, 469–477.
- European Parliament. Directive 2009/32/EC of the European Parliament and of the Council on the approximation of the laws of the Member States on extraction solvents used in the production of foodstuffs and food ingredients. Off. J. Eur. Union 2009, 141, 11.
- Ran, L.; Yang, C.; Xu, M.; Yi, Z.; Ren, D.; Yi, L. Enhanced aqueous two-phase extraction of proanthocyanidins from grape seeds by using ionic liquids as adjuvants. Sep. Purif. Technol. 2019, 226, 154–161.
- Ho, K.; Ferruzzi, M.G.; Liceaga, A.M.; San Martín-González, M.F. Microwave-assisted extraction of lycopene in tomato peels: Effect of extraction conditions on all-trans and cis-isomer yields. LWT 2015, 62, 160–168.
- Ozturk, B.; Winterburn, J.; Gonzalez-Miquel, M. Orange peel waste valorisation through limonene extraction using bio-based solvents. Biochem. Eng. J. 2019, 151, 107298.
- Routray, W.; Orsat, V. MAE of phenolic compounds from blueberry leaves and comparison with other extraction methods. Ind. Crop. Prod. 2014, 58, 36–45.
- Osojnik Črnivec, I.G.; Skrt, M.; Šeremet, D.; Sterniša, M.; Farčnik, D.; Štrumbelj, E.; Poljanšek, A.; Cebin, N.; Pogačnik, L.; Smole Možina, S.; et al. Waste streams in onion production: Bioactive compounds, quercetin and use of antimicrobial and antioxidative properties. Waste Manag. 2021, 126, 476–486.
- Tavares, C.S.; Martins, A.; Miguel, M.G.; Carvalheiro, F.; Duarte, L.C.; Gameiro, J.A.; Figueiredo, A.C.; Roseiro, L.B. Bioproducts from forest biomass II. Bioactive compounds from the steam-distillation by-products of Cupressus lusitanica Mill. and Cistus ladanifer L. wastes. Ind. Crop. Prod. 2020, 158, 112991.
- Wang, T.; Jiao, J.; Gai, Q.-Y.; Wang, P.; Guo, N.; Niu, L.-L.; Fu, Y.-J. Enhanced and green extraction polyphenols and furanocoumarins from Fig (Ficus carica L.) leaves using deep eutectic solvents. J. Pharm. Biomed. Anal. 2017, 145, 339–345.
- Živković, J.; Šavikin, K.; Janković, T.; Ćujić, N.; Menković, N. Optimization of ultrasound-assisted extraction of polyphenolic compounds from pomegranate peel using response surface methodology. Sep. Purif. Technol. 2018, 194, 40–47.
- Allison, B.J.; Simmons, C.W. Valorization of tomato pomace by sequential lycopene extraction and anaerobic digestion. Biomass Bioenergy 2017, 105, 331–341.
- Amyrgialaki, E.; Makris, D.P.; Mauromoustakos, A.; Kefalas, P. Optimisation of the extraction of pomegranate (Punica granatum) husk phenolics using water/ethanol solvent systems and response surface methodology. Ind. Crop. Prod. 2014, 59, 216–222.
- Rahimi, S.; Mikani, M. Lycopene green ultrasound-assisted extraction using edible oil accompany with response surface methodology (RSM) optimization performance: Application in tomato processing wastes. Microchem. J. 2019, 146, 1033–1042.
- Kehili, M.; Sayadi, S.; Frikha, F.; Zammel, A.; Allouche, N. Optimization of lycopene extraction from tomato peels industrial by-product using maceration in refined olive oil. Food Bioprod. Process. 2019, 117, 321–328.
- Goldsmith, C.D.; Vuong, Q.V.; Stathopoulos, C.E.; Roach, P.D.; Scarlett, C.J. Ultrasound increases the aqueous extraction of phenolic compounds with high antioxidant activity from olive pomace. LWT 2018, 89, 284–290.
- Chong, K.Y.; Stefanova, R.; Zhang, J.; Brooks, M.S.-L. Aqueous two-phase extraction of bioactive compounds from haskap leaves (Lonicera caerulea): Comparison of salt/ethanol and sugar/propanol systems. Sep. Purif. Technol. 2020, 252, 117399.
- Dorđević, T.; Antov, M. Ultrasound assisted extraction in aqueous two-phase system for the integrated extraction and separation of antioxidants from wheat chaff. Sep. Purif. Technol. 2017, 182, 52–58.
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Green extraction of grape skin phenolics by using deep eutectic solvents. Food Chem. 2016, 200, 159–166.
- Bosiljkov, T.; Dujmić, F.; Cvjetko Bubalo, M.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Radojčić Redovniković, I.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203.
- Chanioti, S.; Tzia, C. Extraction of phenolic compounds from olive pomace by using natural deep eutectic solvents and innovative extraction techniques. Innov. Food Sci. Emerg. Technol. 2018, 48, 228–239.
- Fernández, M.d.L.Á.; Espino, M.; Gomez, F.J.V.; Silva, M.F. Novel approaches mediated by tailor-made green solvents for the extraction of phenolic compounds from agro-food industrial by-products. Food Chem. 2018, 239, 671–678.
- Huang, Y.; Feng, F.; Jiang, J.; Qiao, Y.; Wu, T.; Voglmeir, J.; Chen, Z.-G. Green and efficient extraction of rutin from tartary buckwheat hull by using natural deep eutectic solvents. Food Chem. 2017, 221, 1400–1405.
- Masci, A.; Coccia, A.; Lendaro, E.; Mosca, L.; Paolicelli, P.; Cesa, S. Evaluation of different extraction methods from pomegranate whole fruit or peels and the antioxidant and antiproliferative activity of the polyphenolic fraction. Food Chem. 2016, 202, 59–69.
- Dahmoune, F.; Spigno, G.; Moussi, K.; Remini, H.; Cherbal, A.; Madani, K. Pistacia lentiscus leaves as a source of phenolic compounds: Microwave-assisted extraction optimized and compared with ultrasound-assisted and conventional solvent extraction. Ind. Crop. Prod. 2014, 61, 31–40.
- Drevelegka, I.; Goula, A.M. Recovery of grape pomace phenolic compounds through optimized extraction and adsorption processes. Chem. Eng. Process.-Process Intensif. 2020, 149, 107845.
- Xi, J.; He, L.; Yan, L.-G. Continuous extraction of phenolic compounds from pomegranate peel using high voltage electrical discharge. Food Chem. 2017, 230, 354–361.
- Klavins, L.; Kviesis, J.; Klavins, M. Comparison of methods of extraction of phenolic compounds from american cranberry (Vaccinium macrocarpon L.). Agron. Res. 2017, 15, 1316–1329.
- Rajha, H.N.; Koubaa, M.; Boussetta, N.; Maroun, R.G.; Louka, N.; Lebovka, N.; Vorobiev, E. Selective ultrasound-assisted aqueous extraction of polyphenols from pomegranate peels and seeds. J. Food Process. Preserv. 2020, 44, e14545.
- Nipornram, S.; Tochampa, W.; Rattanatraiwong, P.; Singanusong, R. Optimization of low power ultrasound-assisted extraction of phenolic compounds from mandarin (Citrus reticulata Blanco cv. Sainampueng) peel. Food Chem. 2018, 241, 338–345.
- Hiranvarachat, B.; Devahastin, S. Enhancement of microwave-assisted extraction via intermittent radiation: Extraction of carotenoids from carrot peels. J. Food Eng. 2014, 126, 17–26.
- Cvetanović, A.; Švarc-Gajić, J.; Zeković, Z.; Mašković, P.; Đurović, S.; Zengin, G.; Delerue-Matos, C.; Lozano-Sánchez, J.; Jakšić, A. Chemical and biological insights on aronia stems extracts obtained by different extraction techniques: From wastes to functional products. J. Supercrit. Fluids 2017, 128, 173–181.
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of subcritical water extraction with natural deep eutectic solvents for sustainable extraction of phenolic compounds from winemaking by-products. Food Res. Int. 2020, 137, 109728.
- Kheirkhah, H.; Baroutian, S.; Quek, S.Y. Evaluation of bioactive compounds extracted from Hayward kiwifruit pomace by subcritical water extraction. Food Bioprod. Process. 2019, 115, 143–153.
- Guthrie, F.; Wang, Y.; Neeve, N.; Quek, S.Y.; Mohammadi, K.; Baroutian, S. Recovery of phenolic antioxidants from green kiwifruit peel using subcritical water extraction. Food Bioprod. Process. 2020, 122, 136–144.
- Grunovaitė, L.; Pukalskienė, M.; Pukalskas, A.; Venskutonis, P.R. Fractionation of black chokeberry pomace into functional ingredients using high pressure extraction methods and evaluation of their antioxidant capacity and chemical composition. J. Funct. Foods 2016, 24, 85–96.
- Espinosa-Pardo, F.A.; Nakajima, V.M.; Macedo, G.A.; Macedo, J.A.; Martínez, J. Extraction of phenolic compounds from dry and fermented orange pomace using supercritical CO2 and cosolvents. Food Bioprod. Process. 2017, 101, 1–10.
- Garcia-Mendoza, M.D.P.; Espinosa-Pardo, F.A.; Baseggio, A.M.; Barbero, G.F.; Maróstica Junior, M.R.; Rostagno, M.A.; Martínez, J. Extraction of phenolic compounds and anthocyanins from juçara (Euterpe edulis Mart.) residues using pressurized liquids and supercritical fluids. J. Supercrit. Fluids 2017, 119, 9–16.
- Loypimai, P.; Moongngarm, A.; Chottanom, P.; Moontree, T. Ohmic heating-assisted extraction of anthocyanins from black rice bran to prepare a natural food colourant. Innov. Food Sci. Emerg. Technol. 2015, 27, 102–110.
- Benito-Román, O.; Varona, S.; Sanz, M.T.; Beltrán, S. Valorization of rice bran: Modified supercritical CO2 extraction of bioactive compounds. J. Ind. Eng. Chem. 2019, 80, 273–282.
- Xiao, C.; Anderson, C.T. Roles of pectin in biomass yield and processing for biofuels. Front. Plant Sci. 2013, 4, 67.
- Chaharbaghi, E.; Khodaiyan, F.; Hosseini, S.S. Optimization of pectin extraction from pistachio green hull as a new source. Carbohydr. Polym. 2017, 173, 107–113.
- Košťálová, Z.; Aguedo, M.; Hromádková, Z. Microwave-assisted extraction of pectin from unutilized pumpkin biomass. Chem. Eng. Process.-Process Intensif. 2016, 102, 9–15.
- Maran, J.P.; Swathi, K.; Jeevitha, P.; Jayalakshmi, J.; Ashvini, G. Microwave-assisted extraction of pectic polysaccharide from waste mango peel. Carbohydr. Polym. 2015, 123, 67–71.
- Hosseini, S.S.; Khodaiyan, F.; Yarmand, M.S. Optimization of microwave assisted extraction of pectin from sour orange peel and its physicochemical properties. Carbohydr. Polym. 2016, 140, 59–65.
- Raji, Z.; Khodaiyan, F.; Rezaei, K.; Kiani, H.; Hosseini, S.S. Extraction optimization and physicochemical properties of pectin from melon peel. Int. J. Biol. Macromol. 2017, 98, 709–716.
- Zannini, D.; Dal Poggetto, G.; Malinconico, M.; Santagata, G.; Immirzi, B. Citrus Pomace Biomass as a Source of Pectin and Lignocellulose Fibers: From Waste to Upgraded Biocomposites for Mulching Applications. Polymers 2021, 13, 1280.
- Tuan, N.T.; Le Dang, N.; Huong, B.T.C.; Danh, L.T. One step extraction of essential oils and pectin from pomelo (Citrus grandis) peels. Chem. Eng. Process.-Process. Intensif. 2019, 142, 107550.
- Ortiz-Sanchez, M.; Solarte-Toro, J.-C.; González-Aguirre, J.-A.; Peltonen, K.E.; Richard, P.; Cardona Alzate, C.A. Pre-feasibility analysis of the production of mucic acid from orange peel waste under the biorefinery concept. Biochem. Eng. J. 2020, 161, 107680.
- Cáceres, L.A.; McGarvey, B.D.; Briens, C.; Berruti, F.; Yeung, K.K.-C.; Scott, I.M. Insecticidal properties of pyrolysis bio-oil from greenhouse tomato residue biomass. J. Anal. Appl. Pyrolysis 2015, 112, 333–340.
- Costa, F.F.; de Oliveira, D.T.; Brito, Y.P.; Rocha Filho, G.N.d.; Alvarado, C.G.; Balu, A.M.; Luque, R.; Nascimento, L.A.S.d. Lignocellulosics to biofuels: An overview of recent and relevant advances. Curr. Opin. Green Sustain. Chem. 2020, 24, 21–25.
- Imam, T.; Capareda, S. Characterization of bio-oil, syn-gas and bio-char from switchgrass pyrolysis at various temperatures. J. Anal. Appl. Pyrolysis 2012, 93, 170–177.
- Urrutia, R.I.; Yeguerman, C.; Jesser, E.; Gutierrez, V.S.; Volpe, M.A.; Werdin González, J.O. Sunflower seed hulls waste as a novel source of insecticidal product: Pyrolysis bio-oil bioactivity on insect pests of stored grains and products. J. Clean. Prod. 2021, 287, 125000.
- Aladin, A.; Yani, S.; Modding, B.; Wiyani, L. Pyrolisis Of Corncob Waste To Produce Liquid Smoke. IOP Conf. Ser. Earth Environ. Sci. 2018, 175, 12020.
- Madhu, P.; Periyanayagi, G. Identification of Bioactive compounds of pyrolysis oil obtained from cotton residues (Gossypium arboreum) by flash pyrolysis. Int. J. ChemTech Res. 2017, 10, 51–66.
- Barbero-López, A.; Chibily, S.; Tomppo, L.; Salami, A.; Ancin-Murguzur, F.J.; Venäläinen, M.; Lappalainen, R.; Haapala, A. Pyrolysis distillates from tree bark and fibre hemp inhibit the growth of wood-decaying fungi. Ind. Crop. Prod. 2019, 129, 604–610.
- Faisal, M.; Gani, A.; Mulana, F. Preliminary assessment of the utilization of durian peel liquid smoke as a natural preservative for mackerel. F1000Research 2019, 8, 240.
- Zhao, Q.; Mäkinen, M.; Haapala, A.; Jänis, J. Valorization of Bark from Short Rotation Trees by Temperature-Programmed Slow Pyrolysis. ACS Omega 2021, 6, 9771–9779.
- Surboyo, M.D.C.; Arundina, I.; Rahayu, R.P.; Mansur, D.; Bramantoro, T. Potential of Distilled Liquid Smoke Derived from Coconut (Cocos nucifera L) Shell for Traumatic Ulcer Healing in Diabetic Rats. Eur. J. Dent. 2019, 13, 271–279.
- Wijaya, M.; Wiharto, M. Synthesis and characterization of bioactive compound from Cocoa fruit shell by pyrolysis process. J. Phys. Conf. Ser. 2020, 1567, 22025.
- Murata, K.; Liu, Y.; Inaba, M.; Takahara, I. Catalytic fast pyrolysis of jatropha wastes. J. Anal. Appl. Pyrolysis 2012, 94, 75–82.
This entry is offline, you can click here to edit this entry!
