Your browser does not fully support modern features. Please upgrade for a smoother experience.
Please note this is an old version of this entry, which may differ significantly from the current revision.
Subjects:
Health Care Sciences & Services
Obesity is a chronic disease characterized by the abnormal or excessive accumulation of body fat. Obesity is commonly associated with other metabolic disorders, such as type 2 diabetes, non-alcoholic fatty liver disease, cardiovascular diseases, chronic kidney disease, and cancers. Factors such as a sedentary lifestyle, overnutrition, socioeconomic status, and other environmental and genetic conditions can cause obesity. Many molecules and signaling pathways are involved in the pathogenesis of obesity.
- obesity
- comorbidities
- molecular signaling pathways
1. Obesity, Its Comorbidities, and Associated Factors
Obesity, directly and indirectly, contributes to many other chronic disorders, including CKD, CVD, NAFLD, and T2D, as well as cancers, such as hepatocellular carcinoma (HCC) [15]. For example, the prevalence of NAFLD in obese patients can be as high as 70–90%, and is positively associated with BMI (≥35) [16]. Inflammation, insulin resistance, and metabolic dysfunction caused by obesity impact the mortality and morbidity of these chronic diseases.
1.1. CKD
CKD is a leading public health problem worldwide, affecting about 13.4% of the world’s population. The most common symptoms in patients with CKD include sleep disturbances, weakness, fatigue, pain, and itchy skin [17]. The progression of CKD can lead to end-stage kidney disease, leading to an increase in renal replacement [18]. Obesity contributes to CKD by increasing intrarenal fat deposition, impaired glomerular filtration rate, and albuminuria [19]. In addition, obesity-associated local and systemic inflammation, insulin resistance, fibrogenesis, and gut microbiota dysbiosis are also associated with CKD development and progression [20,21,22]. Overweight and obese persons with metabolic disorders are more likely to develop CKD [23]. Thus, body weight control and a healthy diet are recommended for obese people.
1.2. CVD
Obesity-associated factors including dyslipidemia, hypertension, insulin resistance, vascular endothelium dysfunction, and sleep disorders can contribute to CVD [24,25]. Obesity-associated comorbidities such as CKD and NAFLD also contribute to CVD [26,27]. For example, obesity-associated, low-grade chronic inflammation in metabolic tissues (e.g., adipose tissues and liver) alters the expression of adipocytokines and lipoproteins such as adiponectin and high-density lipoproteins (HDLs), which influences energy metabolism and causes endothelial dysfunction to increase the risk of CVD [28]. In addition, adipose tissues can secrete many other adipocytokines, such as leptin, resistin, visfatin, TNF-α, and IL-6 [29]. Their roles vary in the pathogenesis of CVD. Adipocytokines such as omentin and adiponectin, which are secreted from visceral adipose tissues (VATs), have anti-inflammatory functions. They can regulate the production of nitric oxide (NO) in endothelial cells and inhibit vascular calcification to prevent atherogenesis and inflammation [30]. In contrast, the expression of resistin and TNF-α contributes to insulin resistance in obesity and T2D. IL-6 is an important cytokine in myocardial lipid accumulation [29]. Blocking the expression of IL-6 or its receptor to the perturb IL-6 signaling pathway results in a reduction in the risk of coronary artery disease and atrial fibrillation, as well as T2D [31].
1.3. NAFLD
NAFLD is the most common type of chronic liver disease, affecting more than 25% of the world population [32,33]. NAFLD is a risk factor for T2D, CVD, and HCC. A large cohort study shows that being overweight and obese are positively and strongly associated with the prevalence of NALFD in metabolically healthy men and women, with multivariable-adjusted average hazard ratios of 2.15 and 3.55, respectively [34]. The prevalence of NAFLD and NASH will increase in multiple countries, as predicted in a model based on the prevalence of obesity and T2D [35]. Many obesity-related factors can contribute to NAFLD and its progression to non-alcoholic steatohepatitis (NASH), including dysfunction of subcutaneous white adipose tissues (scWAT) [36], insulin resistance [37], inflammation [38], dysbiosis of gut microbiota [39], and imbalance in energy metabolism [40].
Although obesity is a contributor to NAFLD, lean NAFLD patients also have a high risk of liver-related death compared to obese and overweight subjects [41]. These lean NAFLD patients do not have visceral fat accumulation (abdominal fat), with less fibrosis and a lower prevalence of T2D compared to obese patients, but commonly have dyslipidemia [42].
1.4. T2D
T2D is a chronic disease with a high level of blood glucose, contributing to the most common type of diabetes. In the comparison of insulin deficiency as a result of pancreatic β-cell damage in type 1 diabetes (T1D), insulin resistance is characterized as the major pathophysiological feature of T2D [43]. T2D affects more than 21.7 million people worldwide, leading to 1.5 million deaths in 2019 [44]. Factors such as physical activity, smoking, diet, and genetic conditions can lead to T2D development and progression. Obesity and insulin resistance can induce pancreatic islet hypertrophy and both α- and β-cell remodeling, disarray, and apoptosis, which can be ameliorated by fasting and anti-T2D treatments [45,46].
1.5. Cancers
Obesity has been identified to promote most cancer progression, including breast cancer [47,48], HCC [49,50], and pancreatic ductal adenocarcinoma (PDAC) [51,52]. Insulin resistance, adipose inflammation, and tumor-promoting growth factors such as fibroblast growth factor 1 (FGF1) are closely associated with most cancer progression [47]. In addition, obesity can impair anti-tumor immunity. For example, obesity-induced hepatic cholesterol accumulation selectively represses the anti-tumor effects of natural killer T (NKT) cells during diet-induced, NAFLD-related HCC [50]. A molecular study further shows that sterol regulatory element-binding protein 2 (SREBP2)-mediated excessive accumulation of cholesterol in hepatocytes causes lipid peroxide accumulation in NKT cells to reduce their cytotoxicity to tumor cells [50].
Overall, obesity can induce chronic inflammation, metabolic syndrome, gut microbiota dysbiosis, and vascular epithelial cell dysfunction to promote the above-mentioned disease progression (Figure 1). Furthermore, obesity also contributes to other diseases, such as COVID-19 infection. One study shows that obesity increases the hospitalization and death rates in children and adolescents who were infected with COVID-19 [53]. The death rate increases in obese patients with a BMI over 40 kg/m2 [54].
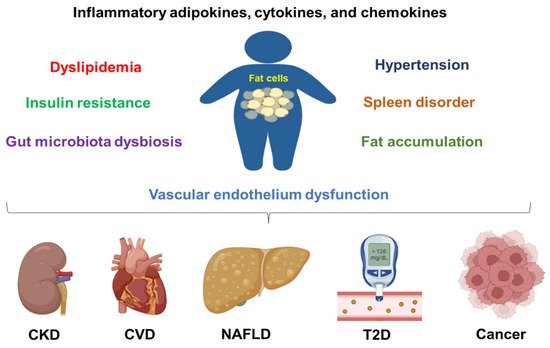
Figure 1. Obesity contributes to chronic disease development and progression. Obesity can cause inflammation, dyslipidemia, insulin resistance, hypertension, fat accumulation, vascular endothelium dysfunction, spleen disorder, and gut microbiota dysbiosis. All these conditions are contributing factors to chronic kidney disease (CKD), cardiovascular diseases (CVDs), non-alcoholic fatty liver disease (NAFLD), type 2 diabetes (T2D), and cancers.
2. Molecular Targets for Obesity Treatment
2.1. Inflammation-Associated Molecules
Elevated serum levels of inflammation markers, such as C-reactive protein (CRP), IL-6, TNF-α, and resistin are commonly found in patients with obesity and who are overweight [55,56,57], accompanying insulin resistance and other metabolic disorders. In contrast, a decreased expression of adiponectin is also found in the same patients. Adipose tissue inflammation is a major pathogenic feature of obesity. The inflammation is contributed to by adipokines, cytokines, and chemokines in adipose tissues, which are secreted from dysfunctional adipocytes and infiltrating immune cells [58]. For example, resistin can be secreted by adipocytes during adipogenesis to cause insulin resistance [57].
Increased vascular permeability and infiltration of leukocytes play an important role in obesity-induced systemic inflammation. For example, the administration of GW311616A, an inhibitor of neutrophil elastase, significantly attenuates vascular leakage, leukocyte infiltration, and the expression of proinflammatory cytokines in the white adipose tissues (WAT) in diet-induced obese mice [59]. The expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1), is associated with the expression of adipogenesis and inflammation markers such as IL-6 and TNF-α [60].
The expression of low molecular weight (15–40 kDa) hyaluronan (HA) molecules is upregulated in the circulating blood of obese individuals, which causes an increased expression of pro-inflammatory cytokines such as IL-1β, IL-8, and monocyte chemoattractant protein-1 (MCP-1, or CCL2) in peripheral blood monocytes [61]. The underlying molecular mechanism study shows that those HAs induce the activation of nuclear factor kappa B (NF-κB) signaling, by regulating the phosphorylation of the α/β complex IκB kinase (IKK).
2.2. Metabolic Syndrome-Associated Molecules
Metabolic syndrome (MetS) is characterized by abdominal obesity, dyslipidemia, hypertension, and insulin resistance [62,63]. Sirtuin 1 (SIRT1), a key metabolic regulator, plays a pivotal role in inflammation and lipid accumulation [64]. SIRT1 regulates the expression of sterol regulatory element-binding transcription factor 1c (SREBF1c) and peroxisome proliferator-activated receptor alpha (PPAR-α) in adipocytes, which play an essential role in the regulation of mitochondrial function [65]. In addition, the expression of molecules including leptin, adiponectin, and matrix metalloproteinases is regulated by SIRT1-mediated signaling pathways [65]. Upregulating the expression of SIRT1 in WAT in mice with high-fat diet (HFD)-induced obesity can ameliorate weight gain [66]. Meanwhile, SIRT1 activation plays a critical role in the inhibition of inflammation, oxidative stress, and cell apoptosis during diseases including obesity, diabetes, and CVDs. For example, an increased expression of SIRT1 in cardiomyocytes is associated with an improvement in insulin sensitivity [67].
2.3. Fatty Acid Metabolism-Associated Molecules
Fatty acid metabolism plays an important role in disease progression, including obesity-associated tumors. Monounsaturated fatty acids can bind G protein-coupled receptors (e.g., GPR120) and peroxisome proliferator-activated receptors (PPARs) to display anti-inflammatory effects by inhibiting macrophage M1 polarization and activating NF-κB and NLR family pyrin domain containing 3 (NLRP3) [68]. In contrast, the intake of medium-chain saturated fatty acids (MCSFAs), but not total saturated fatty acids (TSFAs), is associated with the risk of obesity or being overweight in Chinese adults, which is found in a study with a median of 11 years of follow-up [69]. A clinical trial (NCT02211612) also shows that the consumption of SFAs causes liver fat accumulation and increased serum levels of ceramides, whereas the consumption of polyunsaturated fatty acids (PUFAs) reduces the levels of ceramides, hyperlipidemia, and liver fat accumulation after excessive energy intake in obese persons [70].
2.4. Immune Checkpoints
The expression of programmed death-ligand 1 (PD-L1) in adipose tissues is found to be positively associated with visceral fat accumulation in obese or overweight persons. Conditional knockout PD-L1 on dendritic cells (DCs) increases weight gain and accelerates dietary obesity in mice [71]. Another study shows that the expression of PD-L1 in WAT in mice is associated with the expression of its receptor programmed cell death 1 (PD-1) in visceral WAT [72]. An increased PD-1 expression is found in type 2 innate lymphoid cells in adipose tissues to impair tissue metabolism, which is associated with the recruitment and activation of PD-L1high M1 macrophages [73].
2.5. Exercise-Associated Signaling Molecules
A clinical study (NCT02753231) shows that a low-to-moderate intensity physical education class (LIPE), or its combination with a high-intensity physical education class, significantly regulates the expression of inflammatory or immune regulatory markers, including C-X-C motif chemokine ligand 13 (CXCL13, or BLC), C-C motif chemokine ligand 5 (CCL5), CCL11, CCL13, CCL18, and FGF6 [74]. A mouse study also shows that exercise reduces the expression of TNF-α and macrophage marker F4/80 in adipose tissues in HFD-fed mice, but it increases the expression of the M2 macrophage marker CD163, indicating phenotypic switching from M1 to M2 macrophages in adipose tissues [75]. Exercise can protect the obesity-induced mitochondrial dysfunction and oxidative stress [76], by altering specific lipid profiles including acylcarnitine, diacylglycerol, ceramide, and cardiolipins [77].
2.6. Toll-like Receptors
Toll-like receptors (TLRs) play essential roles in immunity, especially in the innate immune response, by recognizing pathogen-associated molecular patterns (PAMPs) from different microbiomes [78]. Some variants of the TLR-2 gene in exons 3 and 4 are reported to be significantly associated with the development of obesity in overweight persons [79]. TLR4 knockout inhibits HFD-induced obesity in young mice (≤6 months); however, TLR4-/- mice develop spontaneous obesity with chronic low-grade inflammation in aged mice (18 months) [80]. Another study also shows that TLR4-/- mice with HFD display a decrease in M1/M2 macrophage ratio, chronic inflammation, oxidative stress, and insulin resistance, with a higher subcutaneous fat/visceral fat ratio [81]. These changes are associated with the reprogramming of mitochondrial metabolism in adipose tissues. Furthermore, TLRs such as TLR4 and TLR9 contribute to obesity-associated metabolic disorders [82,83].
2.7. Intestinal Barrier-Associated Molecules
Gut microbiota plays a critical role in obesity [84,85]. Leakage of the gut barrier is commonly found during gut microbiota dysbiosis. Some gut microbial species such as a mucin-degrading bacterium Akkermansia muciniphila are involved in the protection of permeabilization of the gut barrier and systemic inflammation [86]. These bacteria also contribute to intestinal stem cell-mediated epithelial cell development and a decreased population of B cells in the intestine [86,87]. In addition, treatment of A. muciniphila can decrease serum levels of inflammatory cytokines (e.g., IL-2 and IFN-γ) and lipid overload, and increase fatty acid oxidation in adipocytes [88].
Overall, many molecules or molecular signaling pathways are involved in the pathogenesis of obesity and its commodities (Figure 2), which are good targets for potential therapies.
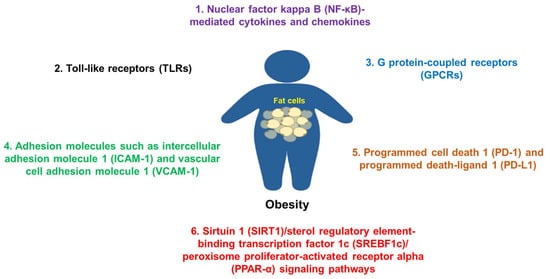
Figure 2. Some common molecules and their signaling pathways in the pathogenesis of obesity. Molecules such as NF-κB, TLRs, adhesion molecules, GPCRs, PD-1/PD-L1, SIRT1, and others are commonly involved in the pathogenesis of obesity.
3. Clinical Management of Obesity
Obesity is a major contributing factor to many diseases at different stages of obesity (Figure 3); therefore, it is critically important to prevent and treat obesity. Some commonly applied strategies in obesity prevention and treatment (Figure 4) are reviewed in this section. In addition, the underlying mechanism of each treatment is also discussed.
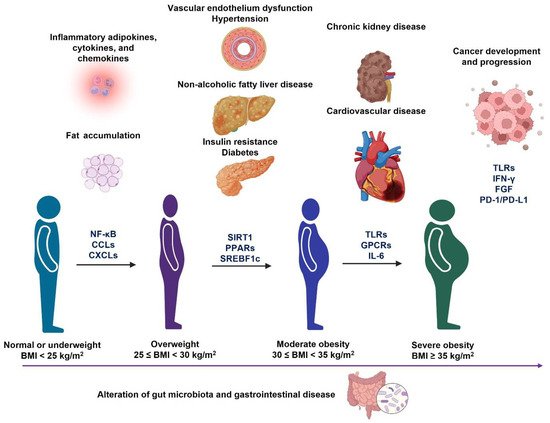
Figure 3. Different metabolic disorders accompany the development and progression of obesity. During the progression of obesity from overweight to severe obesity, it may cause different metabolic disorders, which are associated with the alteration of gut microbiota profiles. In addition, the molecular signaling pathways (Figure 2) may also change during the progression of each obesity-associated comorbidity.
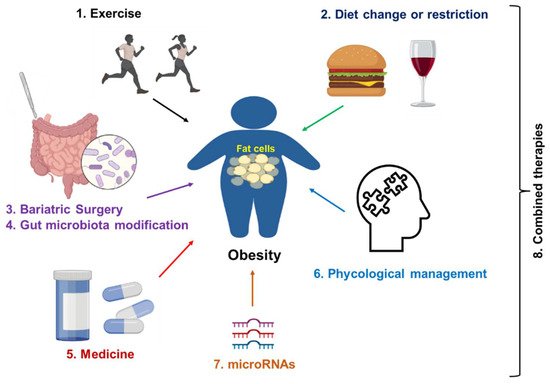
Figure 4. Strategies for obesity treatment. Exercise and dietary change or restriction are commonly applied to prevent and treat the early stage of obesity. Bariatric surgery is a commonly applied treatment for server obesity. Medicines such as semaglutide and liraglutide can be used as monotherapy or a synergistic treatment together with lifestyle management. In addition, psychological management, especially for patients with obesity and distress, is a good option. Gene regulation (microRNAs-mediated therapies) will be applied in the future. Finally, a combination of two therapies or multiple treatments from therapies 1 to 7 can be applied to improve the treatment efficacy.
3.1. Exercise
A meta-analysis study shows that exercise, especially aerobic exercise, significantly decreases the N-terminal-pro hormone B-type natriuretic peptide (BNP) in patients with heart failure, regardless of obesity status [89]. A 12 week exercise program combining aerobic and resistance training on obese β-amyloid-treated rats significantly alleviated the side effects of β-amyloid plaque on brain function, and improved cardiopulmonary function, muscular endurance, and short-term memory ability [90]. This exercise training also activated the expression of many proteins that are associated with brain function in the hippocampus and cerebral cortex, including peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), fibronectin type III domain-containing protein 5 (FNDC5), and brain-derived neurotrophic factor (BDNF).
3.2. Diets
3.2.1. Carbohydrate-Restricted Diets
High-carbohydrate diets usually contain carbohydrates accounting for more than 45% of total calories [91]. Carbohydrate-restricted diets can be classified as moderately low carbohydrate diets (MCD), low carbohydrate diets (LCD), and very low carbohydrate diets (VLCD), with carbohydrates accounting for 26–45%, 10–25%, and less than 10% of total caloric intake, respectively. The MCD and LCD are recommended for adults with obesity or who are overweight as a dietary regimen for weight loss in South Korea [92]. A recent clinical trial study (NCT03814694) shows that weight reduction induced by moderate dietary carbohydrate restriction has no clinically important impact on health-related quality of life and global cognition in obese or overweight patients with T2D [93].
3.2.2. Low-Fat Diet
Treatment with a low-fat diet (LFD) in obese patients reduces the total cholesterol (TC) but not triglycerides (TG). LFD plus moderate-intensity aerobic exercise training (MIAET) significantly decreases both TC and TG and improves depression status, showing greater advantages compared to single treatment alone [94].
3.2.3. Fiber Diet
Dietary fiber, a complex mixture of molecules mainly including polysaccharides, plays a pivotal role in the reduction in obesity and its comorbidities [95]. The underlying mechanisms show that the consumption of a high fiber diet can alter gut hormone secretion (e.g., glucagon-like peptide-1 or GLP-1 and peptide YY or PYY), appetite, secondary bile acid production, energy metabolism, and insulin sensitivity. Twelve week consumption of rye products, a high fiber product with whole grains, causes a greater weight loss and body fat loss, as well as a reduction in C-reactive protein, than the wheat group [96].
3.2.4. Mediterranean Diet
The Mediterranean diet (MedDiet), which consists of unsaturated fats, whole grains, fruits and vegetables, fish, nuts, and legumes, displays the function to reduce bodyweight, and improve obesity and its comorbidities [97,98,99]. For example, a clinical study (NCT04845373) compares the effect of MedDiet with LFD on obese adolescents with NAFLD. MedDiet consists of target macronutrient energy, with 40% from carbohydrates, 35–40% from fats (with <10% of energy as saturated fats), and 20% of energy as proteins, as well as consumption of fish and legumes at least 2–3 times a week, and walnuts and olive oil every day. LFD is composed of target macronutrient energy, with 50–60% from carbohydrates, <30% from fats (with <10% of energy as saturated fat), and 20% from proteins [99]. After a 12 week consumption of MedDiet and LFD, both groups show a significant decrease in hepatic steatosis, serum transaminase levels, and insulin resistance. Subjects in the MedDiet group have lower levels of aspartate aminotransferase (AST) and increased total serum antioxidant ability, with increased levels of paraoxanase-1 and glutathione peroxidase compared to those in the LFD group (p < 0.05). In addition, the MedDiet decreases the CRP levels, while LFD treatment decreases IL-6 compared to basal levels. Another meta-analysis also shows that MedDiet adherence is negatively associated with overweight and/or obesity risk and 5 year weight gain in adults [100]. Overall, appropriate energy limitation and the consumption of low-fat and high-fiber diets improve obesity-related metabolic disorders.
3.3. Bariatric Surgery
Bariatric surgery (BS) is shown to be successful in the treatment of obesity and its comorbidities and the improvement of the quality of life in patients with severe obesity (BMI ≥ 35) [101]. The weight loss outcomes such as total weight loss and final BMI impact the quality of life after bariatric surgery [102]. Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) are the two most commonly used BS procedures for the treatment of obesity and its comorbidities [103]. For example, laparoscopic gastric bypass or SG surgeries significantly decrease BMIs and improve blood glucose levels postprandial in overweight or obese patients with T2D [104]. Some studies show that RYGB is more effective in weight loss than SG in the treatment of obese patients with T2D, but the T2D remission rates are similar post both procedures [105,106,107].
The underlying mechanisms of BS in the improvement of obesity and its comorbidities include the reduction of energy absorption, increase in gut hormone secretion, and improvement of gut microbiota balance. Both RYGB and SG treatments can alter gut hormones to achieve weight loss, such as PYY, GLP-1, and ghrelin. A study shows that RYGB increases blood levels of PYY and GLP-1 at both 26 and 52 weeks after surgery compared to baseline, while SG increases PYY and GLP-1 at 26 weeks, but not at 52 weeks, and decreases blood ghrelin levels in patients compared to baseline [108]. Different levels of these gut hormones induced by BS procedures may have different effects on weight loss. An analysis of gut microbiota profiles in obese patients after bariatric surgery shows that the abundance of genus Blautia is decreased and the abundance of genus Bacteroides is increased after BS, and the ratio of Blautia/Bacteroides is positively associated with BMI [109]. Overall, BS can restrict or reduce energy absorption, increase the secretion of gut hormones, and balance the gut microbiota profile to treat obesity.
3.4. Medicines
Currently, there are anti-obesity medications approved by the Food and Drug Administration (FDA) in the United States, including (1) noradrenergic agonists phentermine, benzphetamine, and phendimetrazine, (2) a serotonin (SE)–norepinephrine (NE)–dopamine (DA)-releasing agent diethylpropion, (3) an opioid receptor antagonist naltrexone with a DA and NE reuptake inhibitor bupropion, (4) a selective 5-hydroxytryptamine 2C (5HT-2C) receptor agonist lorcaserin, (5) a gastrointestinal and pancreatic lipase inhibitor orlistat, (6) GLP-1 analog liraglutide, (7) a GLP-1 receptor agonist semaglutide, and (8) a melanocortin-4 receptor (MC4R) agonist setmelanotide, as well as (9) phentermine with a gamma-aminobutyric acid (GABA) agonist topiramate extended-release [110,111]. The underlying mechanisms of these molecules were reviewed in a previous paper [111]. In addition, some of these are less prescribed by doctors, or have been withdrawn (e.g., diethylpropion in Europe) due to their side effects. Many treatment options are currently under clinical trial evaluation. For example, one single-blinded study shows that a subcutaneous infusion of hormones including PYY, GLP-1, and oxyntomodulin (OXM) at a dose of 4/4/0.4 pmol/kg/min for 4 weeks significantly reduces body weight and improves glucose tolerance in patients with obesity and diabetes [112].
3.5. Management of Gut Microbiota
Gut microbiota plays an important role in many metabolic disorders [15,124,125], including obesity. Mechanistic studies show that gut microbiota can impact diet metabolism and energy balance, gut permeability, intestinal hormone secretion, insulin resistance, and systemic inflammation [126,127]. All these factors are associated with obesity-induced comorbidities. Many factors can cause an alteration of the gut microbiota profile to impact obesity, including diet [128], BS [109], physical activity [129,130], probiotics (e.g., Bifidobacterium and Lacticaseibacillus) [131,132], prebiotics, and synbiotics (containing both probiotic and prebiotic components).
A single U.S. academic medical center clinical trial (NCT02530385) shows that weekly administration of fecal microbiota transplantation (FMT) capsules from healthy lean donors in adults with obesity for 12 weeks results in gut microbiota engraftment in most recipients, without causing adverse events [133]. Another single-center study (NCT02637115) shows that daily oral supplementation of either live or pasteurized A. muciniphila (1010 colony-forming units or CFUs) for three months is well-tolerated and safe [134]. Treatment with pasteurized A. muciniphila significantly improves insulin sensitivity and decreases plasma total cholesterol, and slightly decreases body weight (p = 0.091) compared to the placebo group. In addition, supplementation of A. muciniphila for three months decreases the expression of blood markers for liver dysfunction and inflammation without changing the gut microbiota profile [134]. A 12 week supplementation of probiotics Lactobacillus curvatus HY7601 and Lactobacillus plantarum KY1032 (1010 CFUs) significantly decreases body weight, visceral fat mass, and waist circumference, but increases the expression of adiponectin compared with the placebo group [135]. This treatment changes the gut microbiota profile, causing an increase in the abundance of the Bifidobacteriaceae and Akkermansiaceae families, and a decrease in the abundance of the Prevotellaceae and Selenomonadaceae families. Supplementation of synbiotics increases the abundance of beneficial bacteria Bifidobacterium and Lactobacillus in the gut. There is a negative association between overtime blood glucose levels and the abundance of Lactobacillus, but there is a positive association between the abundance of Bifidobacterium and overtime obesity features including body mass, BMI, waist circumstance, and body fat mass [136].
Additionally, the common BS procedures RYGB and SG can reduce body weight and improve obesity-associated inflammation and insulin resistance by altering gut microbiota (e.g., reduction in genera Butyriciccocus, Eubacterium ventriosum, and Monoglobus) [137]. Another study shows that the consumption of high-fiber rye foods causes an increase in the abundance of butyrate-producing Agathobacter, and a reduction in the abundance of Ruminococcus, which is associated with a reduction in low-grade inflammation and an increase in plasma butyrate [138]. Overall, current clinical studies show the promising effects of different gut microbial intervention strategies on obesity and its comorbidities. A large scale of clinical trials and different compositions of gut microbial species should be investigated in the future for evaluating the efficacy of gut-microbiota-mediated therapies against obesity.
3.6. Psychological Management
Psychological interventions are shown to be effective in the treatment of emotional or mental disorders. Depression is also associated with the development of obesity and its comorbidities [139]. For example, a study in German adults shows that the prevalence of comorbid depression and obesity in men is 1.3% (95% confidence interval/CI: 0.8–2.0), which increases to 2.0% (95% CI: 1.3–3.0) in women [140]. Another cohort study reveals that middle-aged and older Chinese people (≥45 years) with comorbid depression and obesity have a higher risk of developing functional disability than those with depression or obesity alone and without both [141]. Therefore, psychological management may benefit the treatment of obesity.
3.7. MicroRNA-Mediated Therapy
MicroRNAs (miRNAs) are a class of small, non-coding RNAs that play important roles in host health and disease [142]. Recent studies also reveal that miRNAs are involved in adipogenesis and obesity [143], which are potential targets for obesity treatment. For example, miR-203 can target an apical sodium-dependent bile acid transporter to regulate bile acid homeostasis and decrease obesity and dyslipidemia [144]. In addition, the expression of circulating miRNAs such as miR-99-5p/100-5p can be regulated by lifestyle intervention, and is associated with a decrease in fat accumulation and an improvement in glucose metabolism in patients with abdominal obesity [145]. Furthermore, some microRNAs, such as miR-29 family members miR-29a-3p, miR-29b-3p, and miR-29c-3p, are associated with different metabolic disorders, including obesity, insulin resistance, and T2D; therefore, they are potential therapeutic and prognostic markers in obesity and obesity-related metabolic disorders [146,147]. Regulation of target miRNAs can be applied to treat obesity (e.g., miR-21 and miR-502-3p) [148,149] and its comorbidities such as NAFLD (e.g., miR-802 and miR-144) [150,151], T2D (e.g., miR-150 and miR-26a) [152,153], and CVD (e.g., miR-181b and miR-126-5p) [154,155].
This entry is adapted from the peer-reviewed paper 10.3390/healthcare10091616
This entry is offline, you can click here to edit this entry!
